Stratospheric Cooling and Tropospheric Warming - Revised
Posted on 18 December 2010 by Bob Guercio
This is a revised version of Stratospheric Cooling and Tropospheric Warming posted on December 1, 2010.
Increased levels of carbon dioxide (CO2) in the atmosphere have resulted in the warming of the troposphere and cooling of the stratosphere which is caused by two mechanisms. One mechanism involves the conversion of translational energy of motion or translational kinetic energy (KE) into Infrared radiation (IR) and the other method involves the absorption of IR energy by CO2 in the troposphere such that it is no longer available to the stratosphere. The former dominates and will be discussed first. For simplicity, both methods will be explained by considering a model of a fictitious planet with an atmosphere consisting of CO2 and an inert gas such as nitrogen (N2) at pressures equivalent to those on earth. This atmosphere will have a troposphere and a stratosphere with the tropopause at 10 km. The initial concentration of CO2 will be 100 parts per million (ppm) and will be increased to 1000 ppm. These parameters were chosen in order to generate graphs which enable the reader to easily understand the mechanisms discussed herein. Furthermore, in keeping with the concept of simplicity, the heating of the earth and atmosphere due to solar insolation will not be discussed. A short digression into the nature of radiation and its interaction with CO2 in the gaseous state follows.
Temperature is a measure of the energy content of matter and is indicated by the translational KE of the particles. A gas of fast particles is at a higher temperature than one of slow particles. Energy also causes CO2 molecules to vibrate but although this vibration is related to the energy content of CO2, it is not related to the temperature of the gaseous mixture. Molecules undergoing this vibration are in an excited state.
IR radiation contains energy and in the absence of matter, this radiation will continue to travel indefinitely. In this situation, there is no temperature because there is no matter.
The energy content of IR radiation can be indicated by its IR spectrum which is a graph of power density as a function of frequency. Climatologists use wavenumbers instead of frequencies for convenience and a wavenumber is defined as the number of cycles per centimeter. Figure 1 is such a graph where the x axis indicates the wavenumber and the y axis indicates the power per square meter per wavenumber. The area under the curve represents the total power per square meter in the radiation.
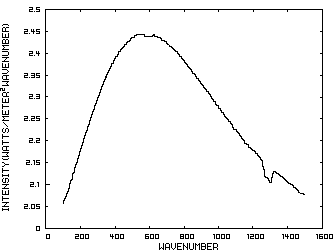
Figure 1. IR Spectrum - No Atmosphere
The interaction of IR radiation with CO2 is a two way street in that IR radiation can interact with unexcited CO2 molecules and cause them to vibrate and become excited and excited CO2 molecules can become unexcited by releasing IR radiation.
Consider now the atmosphere of our fictitious model. As depicted in Step 1 of Figure 2, N2 and CO2 molecules are in motion and the average speed of these molecules is related to the temperature of the stratosphere. Now imagine that CO2 molecules are injected into the atmosphere causing the concentration of CO2 to increase. These molecules will then collide with other molecules of either N2 or CO2 (Step 2) and some of the KE of these particles will be transferred to the CO2 resulting in excited CO2 molecules (Step 3) and a lowered stratospheric temperature. All entities, including atoms and molecules, prefer the unexcited state to the excited state. Therefore, these excited CO2 molecules will deexcite and emit IR radiation (Step 4) which, in the rarefied stratosphere, will simply be radiated out of the stratosphere. The net result is a lower stratospheric temperature. This does not happen in the troposphere because, due to higher pressures and shorter distances between particles, any emitted radiation gets absorbed by another nearby CO2 molecule.
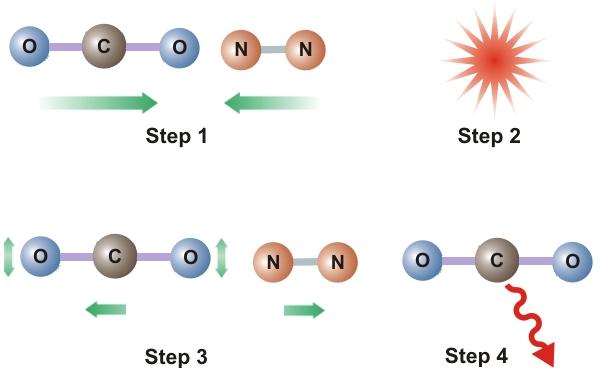
Figure 2. Kinetic To IR Energy Transfer
In order to discuss the second and less dominant mechanism, consider Figure 1 which shows the IR spectrum from a planet with no atmosphere and Figures 3 which shows the IR spectrums from the same planet with CO2 levels of 100 ppm and 1000 ppm respectively. These graphs were generated from a model simulator at the website of Dr. David Archer, a professor in the Department of the Geophysical Sciences at the University of Chicago and edited to contain only the curves of interest to this discussion. As previously stated, these parameters were chosen in order to generate graphs which enable the reader to easily understand the mechanism discussed herein.
The curves of Figures 3 approximately follow the intensity curve of Figure 1 except for the missing band of energy centered at 667 cm-1. This band is called the absorption band and is so named because it represents the IR energy that is absorbed by CO2. IR radiation of all other wavenumbers do not react with CO2 and thus the IR intensity at these wavenumbers is the same as that of Figure 1. These wavenumbers represent the atmospheric window which is so named because the IR energy radiates through the atmosphere unaffected by the CO2.
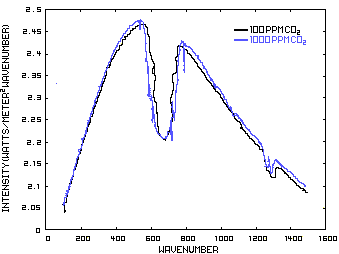
Figure 3. CO2 IR Spectrum - 100/1000 ppm
A comparison of the curves in Figure 3 shows that the absorption band at 1000 ppm is wider than that at 100 ppm because more energy has been absorbed from the IR radiation by the troposphere at a CO2 concentration of 1000 ppm than at a concentration of 100 ppm. The energy that remains in the absorption band after the IR radiation has traveled through the troposphere is the only energy that is available to interact with the CO2 of the stratosphere. At a CO2 level of 100 ppm there is more energy available for this than at a level of 1000 ppm. Therefore, the stratosphere is cooler because of the higher level of CO2 in the troposphere. Additionally, the troposphere has warmed because it has absorbed the energy that is no longer available to the stratosphere.
In concluding, this paper has explained the mechanisms which cause the troposphere to warm and the stratosphere to cool when the atmospheric level of CO2 increases. The dominant mechanism involves the conversion of the energy of motion of the particles in the atmosphere to IR radiation which escapes to space and the second method involves the absorption of IR energy by CO2 in the troposphere such that it is no longer available to the stratosphere. Both methods act to reduce the temperature of the stratosphere.
*It is recognized that a fictitious planet as described herein is a physical impossibility. The simplicity of this model serves to explain a concept that would otherwise be more difficult using a more complex and realistic model.
Robert J. Guercio - December 18, 2010































 Arguments
Arguments























 0
0  0
0






Comments