Toxic mercury, accumulating in the Arctic, springs from a hidden source
Posted on 3 June 2012 by John Hartz
This is a reprint of a news release posted by Harvard University on May 21, 2012
![]()
Harvard study finds circumpolar rivers most responsible for high levels of mercury in the Arctic

The Lena River delta. The Lena is one of several major rivers that flows northward into the Arctic Ocean. (False-color satellite image courtesy of NASA.)
Environmental scientists at Harvard have discovered that the Arctic accumulation of mercury, a toxic element, is caused by both atmospheric forces and the flow of circumpolar rivers that carry the element north into the Arctic Ocean.
While the atmospheric source was previously recognized, it now appears that twice as much mercury actually comes from the rivers.
The revelation implies that concentrations of the toxin may further increase as climate change continues to modify the region's hydrological cycle and release mercury from warming Arctic soils.
"The Arctic is a unique environment because it's so remote from most anthropogenic (human-influenced) sources of mercury, yet we know that the concentrations of mercury in Arctic marine mammals are among the highest in the world," says lead author Jenny A. Fisher, a postdoctoral fellow in Harvard's Atmospheric Chemistry Modeling Group and the Department of Earth and Planetary Sciences (EPS). "This is dangerous to both marine life and humans. The question from a scientific standpoint is, where does that mercury come from?"
The results of the study, which was led jointly by Harvard School of Engineering and Applied Sciences (SEAS) and Harvard School of Public Health (HSPH), appeared in the journal Nature Geoscience on May 20.
Mercury is a naturally occurring element that has been enriched in the environment by human activities such as coal combustion and mining. When converted to methylmercury by microbial processes in the ocean, it can accumulate in fish and wildlife at concentrations up to a million times higher than the levels found in the environment.
"In humans, mercury is a potent neurotoxin," explains co-principal investigator Elsie M. Sunderland, Mark and Catherine Winkler Assistant Professor of Aquatic Science at HSPH. "It can cause long-term developmental delays in exposed children and impair cardiovascular health in adults."
Mercury is considered a persistent bioaccumulative toxin because it remains in the environment without breaking down; as it travels up the food chain, from plankton to fish, to marine mammals and humans, it becomes more concentrated and more dangerous.
"Indigenous people in the Arctic are particularly susceptible to the effects of methylmercury exposure because they consume large amounts of fish and marine mammals as part of their traditional diet," Sunderland says. "Understanding the sources of mercury to the Arctic Ocean and how these levels are expected to change in the future is therefore key to protecting the health of northern populations."
Sunderland supervised the study with Daniel Jacob, Vasco McCoy Family Professor of Atmospheric Chemistry and Environmental Engineering at SEAS, where Sunderland is also an affiliate.
Mercury enters the Earth's atmosphere through emissions from coal combustion, waste incineration, and mining. Once airborne, it can drift in the atmosphere for up to a year, until chemical processes make it soluble and it falls back to the ground in rain or snow. This deposition is spread worldwide, and much of the mercury deposited to Arctic snow and ice is re-emitted to the atmosphere, which limits the impact on the Arctic Ocean.
"That's why these river sources are so important," says Fisher. "The mercury is going straight into the ocean."
The most important rivers flowing to the Arctic Ocean are in Siberia: the Lena, the Ob, and the Yenisei. These are three of the 10 largest rivers in the world, and together they account for 10% of all freshwater discharge to the world's oceans. The Arctic Ocean is shallow and stratified, which increases its sensitivity to input from rivers.
Previous measurements had shown that the levels of mercury in the Arctic lower atmosphere fluctuate over the course of a year, increasing sharply from spring to summer. Jacob, Sunderland, and their team used a sophisticated model (GEOS-Chem) of the conditions in the Arctic Ocean and atmosphere to investigate whether variables like melting ice, interactions with microbes, or the amount of sunlight (which affects chemical reactions) could account for the difference.
Incorporating those variables, however, was not enough.
The GEOS-Chem model, which is backed by rigorous environmental observations and more than a decade of scientific review, quantifies the complex nuances of the ocean-ice-atmosphere environment. It takes into account, for example, ocean mixing at various depths, the chemistry of mercury in the ocean and the atmosphere, and the mechanisms of atmospheric deposition and re-emission.
When the Harvard team adapted it for their Arctic mercury simulations, the only adjustment that could explain the spike in summertime concentrations was the incorporation of a large source to the Arctic Ocean from circumpolar rivers. This source had not been recognized previously.
As it turns out, approximately twice as much mercury in the Arctic Ocean originates from the rivers as from the atmosphere.
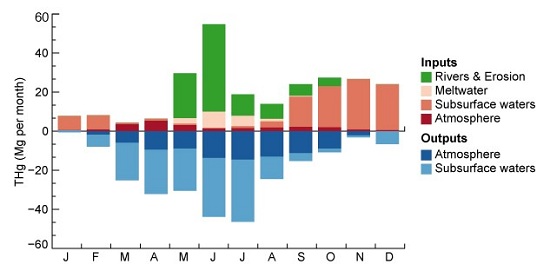
The researchers' new model describes the known inputs and outputs of mercury to the Arctic Ocean. (Image courtesy of Jenny Fisher.)
"At this point we can only speculate as to how the mercury enters the river systems, but it appears that climate change may play a large role," says Jacob. "As global temperatures rise, we begin to see areas of permafrost thawing and releasing mercury that was locked in the soil; we also see the hydrological cycle changing, increasing the amount of runoff from precipitation that enters the rivers."
"Another contributing factor," he adds, "could be runoff from gold, silver, and mercury mines in Siberia, which may be polluting the water nearby. We know next to nothing about these pollution sources."
As the contaminated river water flows into the Arctic Ocean, Jacob says, the surface layer of the ocean becomes supersaturated, leading to what scientists call an "evasion" of mercury from the ocean into the lower atmosphere.
"Observing that telltale supersaturation, and wanting to explain it, is what initially motivated this study," says Fisher. "Relating it to Arctic rivers was detective work. The environmental implications of this finding are huge. It means, for example, that climate change could have a very large impact on Arctic mercury, larger than the impact of controlling emissions to the atmosphere. More work is needed now to measure the mercury discharged by rivers and to determine its origin."
Fisher, Jacob, and Sunderland were joined on this work by co-authors Anne L. Soerensen, a research fellow at SEAS and HSPH; Helen M. Amos, a graduate student in EPS; and Alexandra Steffen, an atmospheric mercury specialist at Environment Canada.
The work was supported by the National Science Foundation's Arctic System Science Program.































 Arguments
Arguments























 0
0  0
0






Comments