Ocean acidification: Global warming's evil twin
Posted on 7 April 2010 by John Cook
While there's much focus on the impacts from warming temperatures, there's another more direct effect from the burning of fossil fuels and deforestation. More than 30% of the carbon dioxide emitted by humans is dissolved into the oceans, gradually turning ocean water more acidic. Coral reef researcher Ove Hoegh-Guldberg explains the threat of ocean acidification: "Evidence gathered by scientists around the world over the last few years suggests that ocean acidification could represent an equal – or perhaps even greater threat – to the biology of our planet than global warming". Thus a new paper Paleo-perspectives on ocean acidification (Pelejero et al 2010) labels ocean acidification the 'evil twin' of global warming.
As CO2 dissolves in the oceans, it leads to a drop in pH. This change in seawater chemistry affects marine organisms and ecosystems in several ways, especially organisms like corals and shellfish whose shells or skeletons are made from calcium carbonate. Today, the surface waters of the oceans have already acidified by an average of 0.1 pH units from pre-industrial levels and we're seeing signs of its impact even in the deep oceans.
The past gives us an insight into future effects of ocean acidification, as we continue to emit more CO2 and acidify the ocean even further. Ice cores give us accurate data on the evolution of CO2 in the atmosphere over the last 800,000 years. These reconstructions, together with data derived from foraminifera, find that the pH of ocean surface water was lower during interglacials (high levels of atmospheric CO2). Seawater pH was also higher during glacial periods when atmospheric CO2 was low. Correspondingly, foraminifera seem to have grown thicker or thinner shells over glacial–interglacial timescales in time with changing CO2 levels.
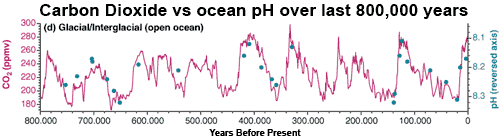
Figure 1: Glacial–interglacial variability in surface water pH (filled blue symbols, note the reversed axis), superimposed on atmospheric CO2 concentration during the last 800,000 years (magenta curve) (Pelejero 2010).
Current atmospheric CO2 are at greater levels than seen over the last 800,000 years. Similarly, pH levels are already more extreme than those experienced by the oceans over this same period. By the end of the 21st century, the projected decline in seawater pH is expected to be three times larger than any change in pH observed as the Earth’s climate has oscillated between glacial and interglacial periods. The times when seawater pH changed fastest was during glacial terminations when the Earth came out of an ice age. The change in seawater pH over the 21st Century is projected to be around 100 times faster than this rate.
What will be the effect of seawater pH falling to such levels? Let's look further back at periods when pH fell to the levels projected for the end of the 21st Century. There have been several periods where pulses of CO2 have been injected into the atmosphere, from volcanic activity or melting of methane hydrates. One well known example is the Paleocene-Eocene Thermal Maximum (PETM), which occurred around 55 million years ago. During this event, global temperatures increased by over 5°C over a time frame less than 10,000 years. This coincided with a massive release of carbon dioxide into the atmosphere, which led to ocean acidification. This change caused a series of biological responses, including the mass extinction of benthic foraminifera.
Looking further back, there are other examples of mass-extinctions coinciding with global warming and increases in atmospheric carbon dioxide. Examination of the mass extinction that occured 251 million years ago during the end-Permian find that the patterns of mortality are consistent with the physiological effects of elevated CO2 concentrations (along with the effects of global warming). 205 million years ago at the Triassic–Jurassic boundary, a sudden rise in the levels of atmospheric CO2 coincided with a major suppression of carbonate sedimentation, very likely related to ocean acidification. A similar situation occurred 65 million years ago during the Cretaceous–Tertiary extinction event. Most of the planktonic calcifying species became rare or disappeared.
Future acidification depends on how much CO2 humans emit over the 21st century. By the year 2100, various projections indicate that the oceans will have acidified by a further 0.3 to 0.4 pH units, more than many organisms like corals can stand. This will create conditions not seen on Earth for at least 40 million years.
A highly recommended website is Climate Shifts, run by one of the coauthors of Pelejero 2010. with a strong emphasis on coral reefs. For more peer-reviewed research on ocean acidification, check out AGW Observer's Papers on Ocean Acidification.































 Arguments
Arguments


























 0
0  0
0 Here is an example of a commonly referenced research of ocean surface pH drops attributed to atmospheric CO2 in a region subject to upwelling - Wootton (2008)
It is vitally important for the cause of sound science to look at all causes for ocean pH changes and accurately represent their relative impacts. Otherwise, this "advocacy science" will cast doubt on the whole community.
Here is an example of a commonly referenced research of ocean surface pH drops attributed to atmospheric CO2 in a region subject to upwelling - Wootton (2008)
It is vitally important for the cause of sound science to look at all causes for ocean pH changes and accurately represent their relative impacts. Otherwise, this "advocacy science" will cast doubt on the whole community.








Comments