CO2 Problems: Parallel concerns breed parallel denial
Posted on 10 November 2011 by chuckbot
You are likely already aware of the CO2 problem: ?trace gasses (primarily carbon dioxide) in the earth’s atmosphere alter its thermal properties, causing it to retain heat. Human activity, primarily the burning of fossil fuels, is increasing the carbon dioxide content of the atmosphere and as a result heating up the earth’s surface. However, a less appreciated fact is that in addition to being a greenhouse gas, carbon dioxide is acidic. This is not at all controversial; it was well recognized more than a century ago in Svente Arhennius’s pioneering article 'On the influence of carbonic acid in the air upon the temperature on the ground’. When we burn fossil fuels, we add CO2 to the atmosphere, but about a quarter that carbon winds up in the oceans. This increases the acidity of the oceans, with potentially severe repercussions for organisms like corals, which build shells out of calcium carbonate and suffer under more acidic conditions. The chemistry is relatively straightforward, and not especially controversial; if you would like more information on the subject, Skeptical Science has an excellent introductory series written by Doug Mackie, Christina McGraw, and Keith Hunter.
When the science of anthropogenic climate change proved politically and economically inconvenient for many people, a cottage industry has popped up in trying to dismiss it. The same effort to dismiss ‘the other CO2 problem’ is now underway; there have been a few snipes from Monckton and the Idsos, but I want to focus on some recent congressional testimony in this vein, coming from a Dr. John Everett. A copy of his testimony can be found here. I think it's important to go through this testimony, because it is one of the only pieces of high-profile misinformation on the subject, and because it shows similar distortions at work behind the denial of climate change and ocean acidification. As you read on, ask yourself: have I seen this tactic before? Graphs manipulated so as to hide an incline? An extrapolation of a few years' worth of climate data? "It's changed in the past"?
What’s a pollutant?
Much of the debate recently has been over the Environmental Protection Agency’s jurisdiction in regulating carbon dioxide; to this end, ‘skeptics’ like Dr. Everett often claim that because it is a natural substance with important natural roles, carbon dioxide is not a pollutant. This is not a very good argument: there are plenty of chemicals which in some situations are essential, but problematic in others. Stratospheric ozone protects me from ultraviolet light, which causes skin cancer. But near the surface, ozone is a component of photochemical smog, interferes with photosynthesis, and causes respiratory illness. Tropane alkaloids are what make deadly nightshade deadly, but they’re the antidote to sarin nerve gas. Hydrochloric acid is important in my stomach, where it helps me digest food- but I don’t want to get it in my eyes.
A pollutant is an ecophysiological poison, and poisons are situational: whether or not a substance is poisonous depends upon its amount, the rate it’s encountered, and other factors. The reality of ocean acidification justifies describing CO2 as a pollutant in the current environmental context.
Pedantic Semantics
Dr. Everett starts off his testimony on a low note, saying,
'Importantly, oceans are alkaline – not acidic, so use of the term “acidification” unnecessarily promotes fear.'
To his credit, this is great PR. It’s snappy, it’s to the point, it sounds nice, it’s easily remembered and repeated. But it’s scientifically vacuous. If a solution has a pH above 7, it is basic. If its pH drops, it has become more acidic- acidified- even if the solution is still basic. If the pH of a solution changes from 9 to 8, the solution has acidified, even though it is still basic. If someone who’s fallen off a bridge says, 'I’m falling doooooooooown’, Dr. Everett’s remark would be like saying, ‘You‘re not falling DOWN! You‘re still way UP in the air!’ The most charitable explanation I can come up with is that he’s confusing the rate of change in a variable (acidification) with the variable itself (acidity). His testimony, we will see, is characterized by this sort of confusion.
Or maybe he just wants to play word games.
Fizzix
‘Something is very wrong’ with our understanding of carbon geochemistry, Dr. Everett declares - therefor we should ‘give the information on its impacts a second look.’ What are his concerns?
First, he revisits ‘the bombshell from Bristol,’ a paper by Wolfgang Knorr which found that the fraction of CO2 emissions which remain in the air hasn’t changed significantly over the past 160 years. Though it does not remotely challenge the AGW consensus, Knorr’s paper was picked up by the ‘skeptic’ blogosphere. Curiously, Everett’s citation for the paper is botched - its cautious title (‘Is the airborne fraction of anthropogenic CO2 increasing?’) has been replaced with a more decisive headline popular amongst the ‘skeptic’ interblogs (‘No Rise of Atmospheric Carbon Dioxide Fraction in Past 160 Years’) What does the paper say? Basically, it finds evidence that the ocean and land haven’t slowed down as carbon sinks - they are still absorbing carbon emissions at the same rate as during the mid 19th century.
It’s hard to see how Knorr’s results support Everett’s opinion that projections of future acidification are overestimates. CO2 emissions have to go somewhere. If the fraction which remains in the air doesn't change, then the fraction which goes other places, like the ocean, can't change either. In fact, if Knorr’s results are correct and the carbon sinks are stable, then ocean acidification will continue unabated because CO2 will continue to flow into the ocean at the present rate.
Next, Dr. Everett claims to find a decline in the rate at which CO2 is building up in the atmosphere. First, he makes the long-term trend in CO2 accumulation rates disappear by stretching his graph of the data to be three times as wide as it is tall:
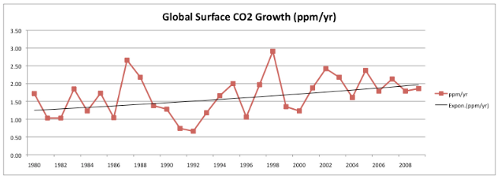
Fig 1. Taken from the written statement of Dr. John Everett before Senate EPW hearing on “EPA's Role in Protecting Ocean Health”, May 11, 2010.
I want to believe that no one would intentionally use such an insultingly transparent trick to hide an incline, but then again there’s this.
Then, he takes the last decade or so of this data, draws a line through it, finds no significant trend, and declares that the quantity is declining. If this sounds familiar, you’re right- do the same thing with HadCRU temperature data and you find that global warming stopped in 1998. It’s amazing!
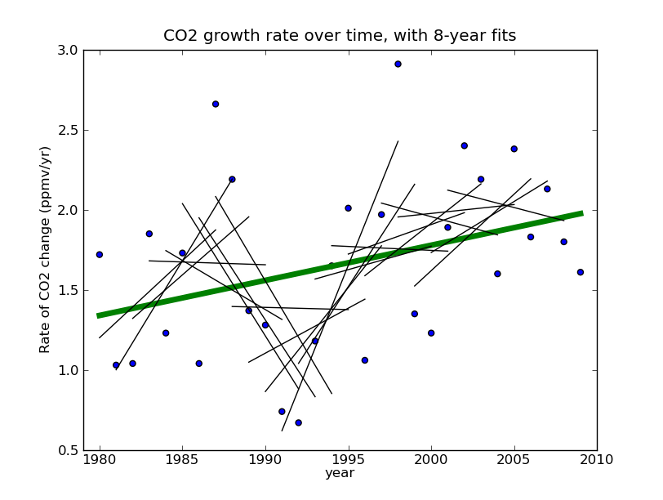
Fig 2. Global CO2 rate data taken from Thomas Conway and Pieter Tans, Annual Mean Carbon Dioxide Growth Rates NOAA/ESRL.
If we apply his technique to the whole data set, we see that 8-12 years are not enough to say much about the longer term trend. Would extrapolating the sharp upswing in 1991-1999 have been a good idea? Would it have been justified? What about the sharp downswing in 1986-1994? It’s pretty clear that projecting patterns from a decade of this data a century into the future is risky at best. And he’s doing it in the face of a clear upward trend at longer timescales!
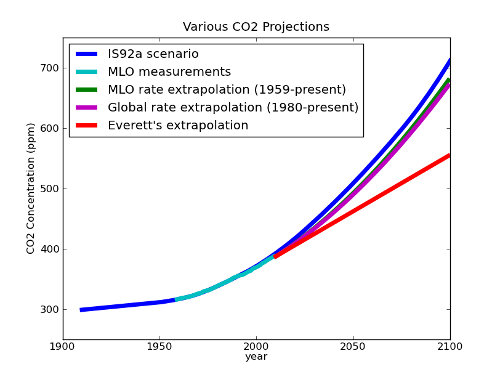
Fig 3. MLO data from Pieter Tans and Ralph Keeling. Mauna Loa Annual Mean Data, NOAA/ESRL/Scripps. IS92a Scenario from IS92a CO2 concentrations. Dr. Everett's projection (described in his remarks in part II) is based upon the assumption of a constant accumulation rate of 1.87 ppm/year; the extrapolations based on MLO (1959-present) and global (1980-present) rate data are calculated by integrating the least-squares linear fit with the 2009 CO2 concentration as an initial condition.
When we extrapolate the trend in the data as a whole, we find that it actually agrees fairly well with IS92a. And even Dr. Everett’s projection entails significant increases in CO2 over the next century, bringing with it significant acidification. Experiments have demonstrated decreased calcification in shellfish at these levels of CO2 (Gazeau et al 2007).
BiLOLogy
Dr. Everett doesn’t trust ocean acidification experiments, though- he claims that ‘the peer review process has warts’. Although he brings up a number of issues, they are all somewhat specious. He claims that experiments have not been carried on long enough to allow organisms to adapt to high-CO2 environments... however, a paper which he describes as ‘Perhaps the most thorough review of the literature on acidificaton impacts’ suggests exactly the opposite: experiments may not be continuing long enough to show the full severity of the problem!
'Although suppression of metabolism under short-term experimental conditions is a "sublethal" reversible process, reductions in growth and reproductive output will effectively diminish the survival of the species on longer time-scales.' (Fabry et al. 2008).
Two of the authors he cites have rebutted, pointing out a number of misrepresentations of their work. Dr. Rodriguez-Iglesias and colleauges found that under high-CO2 conditions, coccolithophores build thicker shells, which is definitely interesting. But does this mean that ‘more CO2 is good’, as Dr. Everett claims? Not really:
‘Dr. Everett’s conclusion that ocean acidification poses no threat to marine organisms is based, in large part, on the Iglesias-Rodriguez et al. (2008) study that showed that calcification within coccolithophores (calcifying phytoplankton) was enhanced under elevated CO2. However, this study also showed that growth rates for these marine algae were simultaneously impaired under high-CO2 conditions.These algae are among the most important sinks of atmospheric CO2 on the planet. Although they release CO2 through calcification, they consume it through photosynthesis (growth). Thus, a shift to enhanced calcification (release of CO2) and reduced growth (consumption of CO2) would substantially reduce the ocean’s ability to sequester CO2 from the atmosphere. [...] The physiology of the whole organism, not simply its ability to calcify, must be investigated to fully assess its ability to survive in a future high-CO2 world.'
The whole thing is worth reading.
Deep Time is Deep
We next hear another common talking point: carbon dioxide levels have changed in the past, so what’s the big deal? To drive the point home, Dr. Everett shows us a graph from the IPCC, showing atmospheric carbon dioxide levels over the last 400 million years. For reference, the magnified section stretches back to before the dinosaurs vanished:
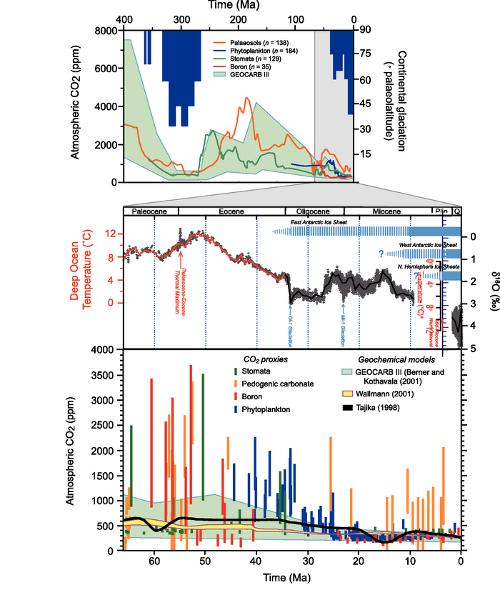
But what he fails to consider is that the rate at which environmental variables change can be as important as those variables themselves. The price of evolution is natural selection, and if natural selection culls individuals faster than they can be replaced, the species is in trouble (as well as the species which depend upon it). This is why rapid environmental changes such as the Paleocene-Eocene Thermal Maximum are associated with mass extinction in the geological record. The faster the environmental change, the stronger the selective pressure - and current rates of environmental change may well exceed organisms’ ability to adapt.
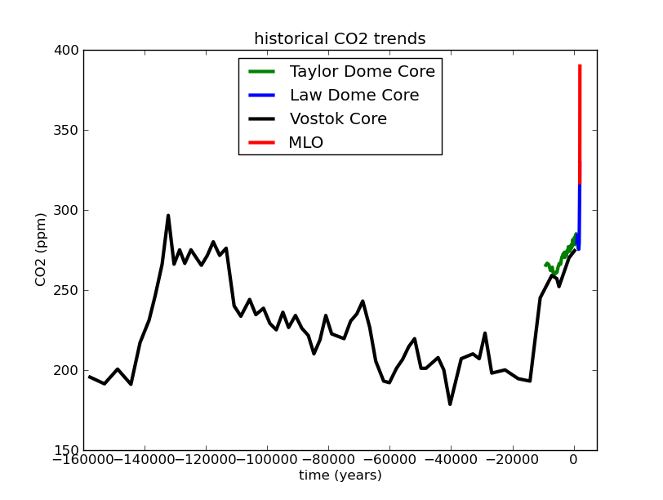
Fig 4. MLO data as with Fig 3; all others from the Paleoclimatology archive of NOAA's National Climatic Data Center. Law Dome data were the 75-year smoothed series from Etheridge, D.M., et al., 2001, Law Dome Atmospheric CO2 Data, IGBP PAGES/World Data Center for Paleoclimatology Data Contribution Series #2001-083. NOAA/NGDC Paleoclimatology Program, Boulder CO, USA. Taylor dome data were from Indermühle, A., B. Stauffer, T.F. Stocker and M. Wahlen, 1999, Taylor Dome Ice Core CO2 Holocene Data. IGBP PAGES/World Data Center-A for Paleoclimatology Data Contribution Series #1999-021 NOAA/NGDC Paleoclimatology Program, Boulder CO, USA. Vostok data were originally published in Barnola, J.-M., D. Raynaud, Y.S. Korotkevich, C. Lorius. 1987. Vostok ice core provides 160,000-year record of atmospheric CO2. Nature 329:408-414; the data were retrieved from the NCDC archives.
Here’s what I mean. If we look at the last glacial cycle, it’s obvious that CO2 has changed over time. The present day is also clearly unusual. But when you consider the rate of CO2 change, our current situation becomes unprecedented.
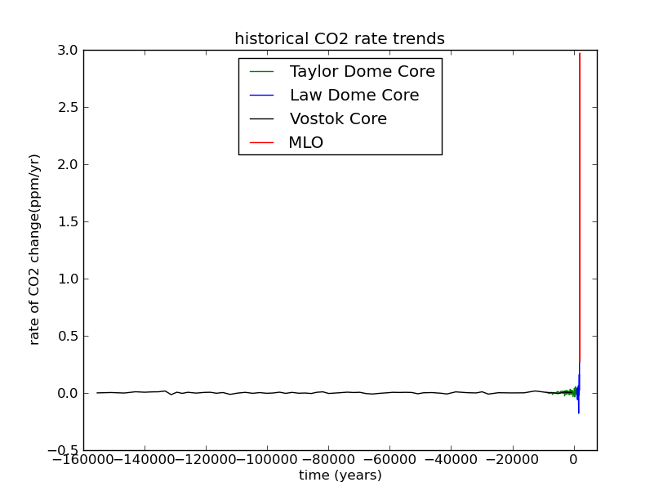
Fig 6: Same data as Figure 5; rates have been calculated by dividing the difference between successive concentrations by the length of time between them, and assigning the resulting rate to the midpoint of the time interval (ie, given a time series [Ti, Xi], a new time series is constructed [ti, Yi] where ti = (Ti + Ti+1)/2 and Yi = (Xi+1 - Xi) / (Ti+1 - Ti))
Endgame
Wrapping up, Dr. Everett grossly misrepresents a news item to make it appear as though acid rain was ultimately not a problem. He suggests, without further support, that, with global temperature 'having reached a peak several years ago, we are at the start of a cooling cycle that will last several decades or more.’ The earth has changed in the past. Genetic plasiticity will magickally intervene to let organisms adapt at warp speed. ‘With no laboratory or observational evidence of biological disruption, I see no economic disruption of commercial and recreational fisheries, nor harm to marine mammals, sea turtles or any other protected species.’
Don’t you feel less alarmed already?
If you are interested in more detail, a thorough debunking of this testimony may be found here.































 Arguments
Arguments























 and even after explaining that the changes are taking place on very different time scales, they still see the current event as being comparable to the 100myear scale oscillations. It would be neat though. I made some fancy backgrounds for some of my graphs and the one I did for the Vostok/Taylor/Law/MLO concentrations was my favorite. I was reading a lot of HP Lovecraft at the time and especially liked the story At the Mountains of Madness, which is set during a geological research expedition to Antarctica. I based it a lot on how that story felt to me
and even after explaining that the changes are taking place on very different time scales, they still see the current event as being comparable to the 100myear scale oscillations. It would be neat though. I made some fancy backgrounds for some of my graphs and the one I did for the Vostok/Taylor/Law/MLO concentrations was my favorite. I was reading a lot of HP Lovecraft at the time and especially liked the story At the Mountains of Madness, which is set during a geological research expedition to Antarctica. I based it a lot on how that story felt to me









[DB] See Skywatcher's response to you below. This is yet another instance of your furnished source not saying what you imply that it does.
For the general reader, please note that the other source Arkadiusz uses, the NIPCC, is discussed here:
Let the Prudent Reader beware.