Ice data made cooler
Posted on 9 December 2010 by jg
Guest post by John Garrett
I am an illustrator who started studying the primary scientific literature to better understand what I thought was a controversy over global warming. I quickly learned that climate science was not an island surrounded by controversy, but a well-founded continent, and most critics I met were merely poor navigators. To point them toward land, I created an interactive Vostok viewer, a Flash program that provides simultaneous displays of temperature, greenhouse gases, incident solar radiation (insolation), and Earth's eccentricity, tilt, and climatic precession for the span of the Vostok ice core.
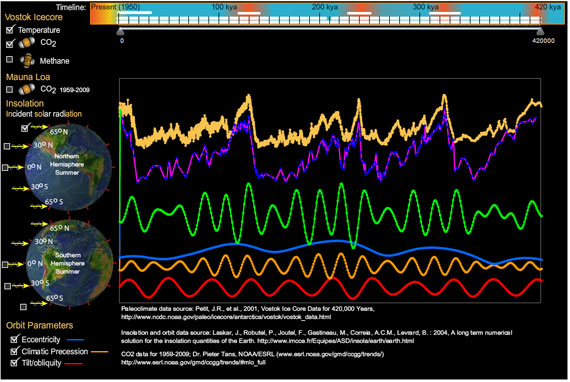
My Vostok viewer uses data from these sources, which deserve the primary credit:
- Petit, J.R., et al., 2001, Vostok Ice Core Data for 420,000 Years, IGBP PAGES/World Data Center for Paleoclimatology Data Contribution Series #2001-076. NOAA/NGDC Paleoclimatology Program, Boulder CO, USA.
- Astronomical Solutions for Earth Paleoclimates. A&A 428, 261-285 (2004), DOI: 10.1051/0004-6361:20041335; Laskar, J., Robutel, P., Joutel, F., Gastineau, M., Correia, A.C.M., Levrard, B. : 2004, A long term numerical solution for the insolation quantities of the Earth.
- CO2 data for 1959-2009; Dr Pieter Tans, NOAA/ESRL
My viewer is a supplement to Earth, Orbit and Climate, an illustrated presentation I created for my astronomy club, which I drew (literally) from Ray Pierrehumbert's Principles of Planetary Climate* (I credit Pierrehumbert but claim no endorsement).
I recently became interested in learning more about the Eemian interglacial and added this to my Earth, Orbit and Climate presentation. I created a variety of graphs and paired them with diagrams of the Earth's changing orbit. For example:
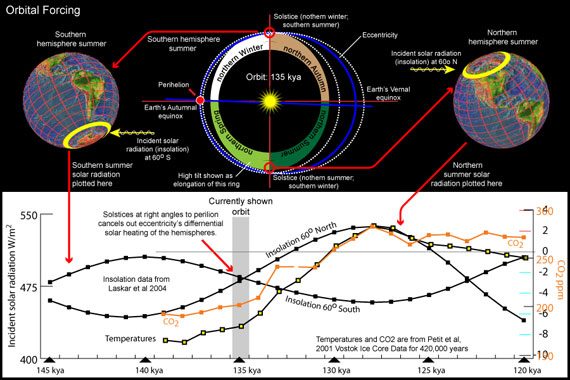
My original goal was to recontruct past orbits (top of this diagram), but seeing the correlation between insolation, CO2, and temperature evoked more curiousity: What about insolation at other latitudes? And at different time periods?
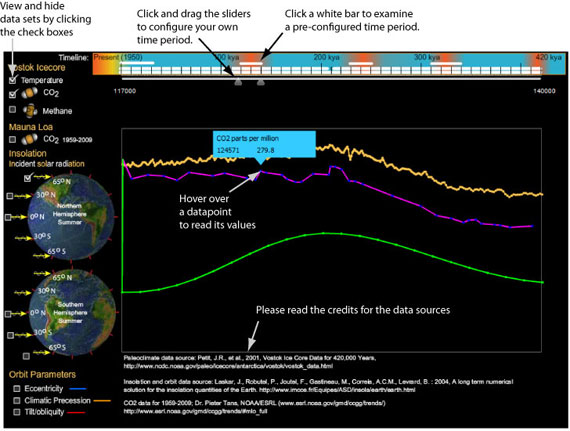
To examine similar correlations for other interglacials, I had to re-export the data, convert it to a table, and then to an illustration. In this process, I was concerned that I would make errors: for example, it would be easy for me to shift one of the graphs left or right, accidentally pairing data from different times (a mistake I made at least once). So in designing my viewer I decided it had to 1) preserve the data for easy verification against my sources, 2) zoom in on any span of time, 3) bring in any dataset that is indexed by date, and 4) fit in 900x600 pixel screen to ensure I can use it in my astronomy presentations. Using the viewer is described below:
Since creating this program I made another personal discovery (that climatologists already knew). Compare northern summer and southern summer insolation at 0 degrees latitude to the same for 65 degrees north and south. Change in insolation at 0 latitude is symmetrical between north and south, but as you approach the poles, this change becomes asymmetrical, hinting at the profound influence in global climate from northern insolation.
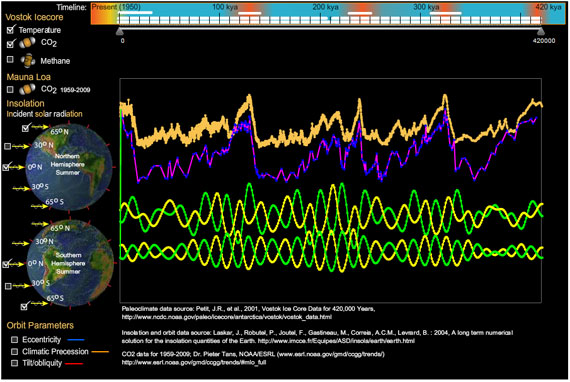
Equally profound is the latest addition of the Mauna Loa CO2 measurements. It challenges the hard-coded vertical scaling I used for the graphs. I will make the scaling more flexible in following versions. I'll also accept requests on features to add.
John Garrett, Wildomar, CA
* Principles of Planetary Climate will be released on Dec 31, 2010 (per Amazon books). I just ordered my copy.































 Arguments
Arguments























 0
0  0
0






Comments