Greenhouse Effect Basics: Warm Earth, Cold Atmosphere
Posted on 29 February 2012 by Tom Curtis
Heating and Heat Flow
Some physics, everyone knows. In our daily lives we encounter the effects of physics all the time, and as a result, we know what physics predicts in those circumstances at a gut level. We may not be able to put it into numbers. We may not be able to apply it in novel situations. But we know it all the same.
One example is as simple as putting on a blanket. We know that if we want warm something up, we can increase the supply of heat - or we can reduce the escape of heat. Either is effective. If you have a pot that is simmering and you want to bring it to the boil, you can turn the heat up, or you can put on the lid. If we put on the lid, the pot will go nicely from simmering to boiling, and we don't need to turn up the heat even slightly. Indeed, if we are not careful to turn down the heat, the pot may well boil over.
Likewise, if you have two identical motors running with an identical load and speed (Revolutions Per Minute), one with the water pump working and one without, we are all physicist enough to say that the second one will run hotter. It does not matter that the energy supplied as fuel is identical in both cases. The fact that heat escapes more easilly with water circulating through the radiator will keep the first cooler. The consequence is that stopping the the water from circulating will lead second motor to disaster.
Nor do we find people who doubt this. Suppose somebody told us their water pump was broken, but that the Second Law of Thermodynamics prohibited transfer of heat from a cooler place (the water) to a hotter place (the engine block), so they'ld be fine so long as they didn't rev any faster than normal, we'ld look at them in complete disbelief. Or we would if we were too polite to burst out laughing. And if they set out cross country confident in their belief, it doesn't matter what destination they claim they're heading for. Rather, as we all know, they're really heading for a breakdown!

(Image copyright to iStock, and not to be reproduced without their permission.)
Heat Flow to Space
This physics that everyone knows is not only true of pots and radiators. It is true of the Earth as well. The Earth is warmed by our remarkably stable Sun. As a result, the Earth's surface radiates energy to space, and over time the incoming energy balances the outgoing energy. The process is made more complicated, however, by the existence of Infra Red (IR) absorbing molecules in the atmosphere.
Without those molecules, Infra Red radiation from the Earth's surface would travel directly to space, cooling the Earth quickly and efficiently. At certain wavelengths of Infra Red radiation, however, those molecules absorb many, or all, of the photons emitted from the Earth's surface. That energy is often redistributed among other molecules by collision, but eventually some of the redistributed energy will be reradiated by the Infra Red absorbing molecules. This process absorption, redistribution and then re-emission may occur many times before the energy escapes the atmosphere, but eventually it will either by being emitted to space, or back to the surface.
Intuitively, the energy that goes through multiple stages of absorption, redistribution and re-emission will not escape to space as fast that which is emitted directly to space from the surface. This intuition is sound, but it depends essentially on one factor, the temperature of the atmosphere.
We can see this by considering a fundamental law that governs the radiation of energy, the Stefan-Boltzmann Law:
![]()
In words, that is J-star equals epsilon sigma T to the fourth power, but we don't need to worry about that. What we need to notice is that J-star, which is the energy radiated over a given time from a given area, is proportional to the fourth power of T, ie, temperature. If the temperature doubles, the energy radiated increases sixteen-fold. If it triples, it increases eighty-one- fold. And so on. So, if the temperature of the atmosphere is different from that of the surface, the absorption, redistribution and re-emission of IR radiation by molecules in the atmosphere will certainly change the rate at which heat escapes to space.
Higher is Colder
There is another piece of physics everyone knows. It is that as you go higher in the atmosphere, the atmosphere gets colder. That is the reason why some mountain peaks are snow covered while their bases are still warm. This is not a universal law. It is not true, for example, in the stratosphere where the absorption of UltraViolet radiation from the Sun causes temperatures to rise with increased height. But eighty percent of the Earth's atmosphere is in the troposphere (the lowest layer of the Earth's atmosphere), and most radiation leaving the top of the troposphere escapes to space. And in the troposphere, as you get higher, the temperature gets lower. On average, the temperature drops by 6.5 degrees C for every thousand meters of altitude you climb. That means, for example, that the temperatures fall by about 24.5 degrees C as you climb to the summit of Mount Fuji, and by 50 to 100 degrees as you rise to the top of the troposphere.

We have already seen that temperature significantly effects the radiation of heat. Colder objects radiate less energy, and the Infra-Red absorbing molecules in the atmosphere are colder than the surface. Therefore it is no surprise that the Infra-Red absorbing molecules in the atmosphere radiate less energy to space than they absorb from the warmer surface. That difference is the essence of the greenhouse effect.
No More Arm Waving
It would be helpfull to recapitulate at this point. So far we have noted four simple facts:
- That if you reduce the escape of heat, but do not reduce the incoming heat, things warm up;
- That the atmosphere contains molecules that absorb Infra-Red radiation;
- That radiated energy depends on the temperature of the radiating object; and
- That the atmosphere gets cooler as you get higher, so that the Infra-Red absorbing molecules in the atmosphere radiate less energy to space than they absorb from the surface.
These four facts imply the existence of an atmospheric greenhouse effect, ie, that the presence of Infra-Red absorbing molecules in the atmosphere results in the surface being warmer than it otherwise would be.
In science, however, purely verbal reasoning like this is considered suspect. The reason is that sometimes odd effects occur that render verbal reasoning moot. So in science, there is no substitute for putting the theory into a mathematical form. It gets rid of the arm waving.
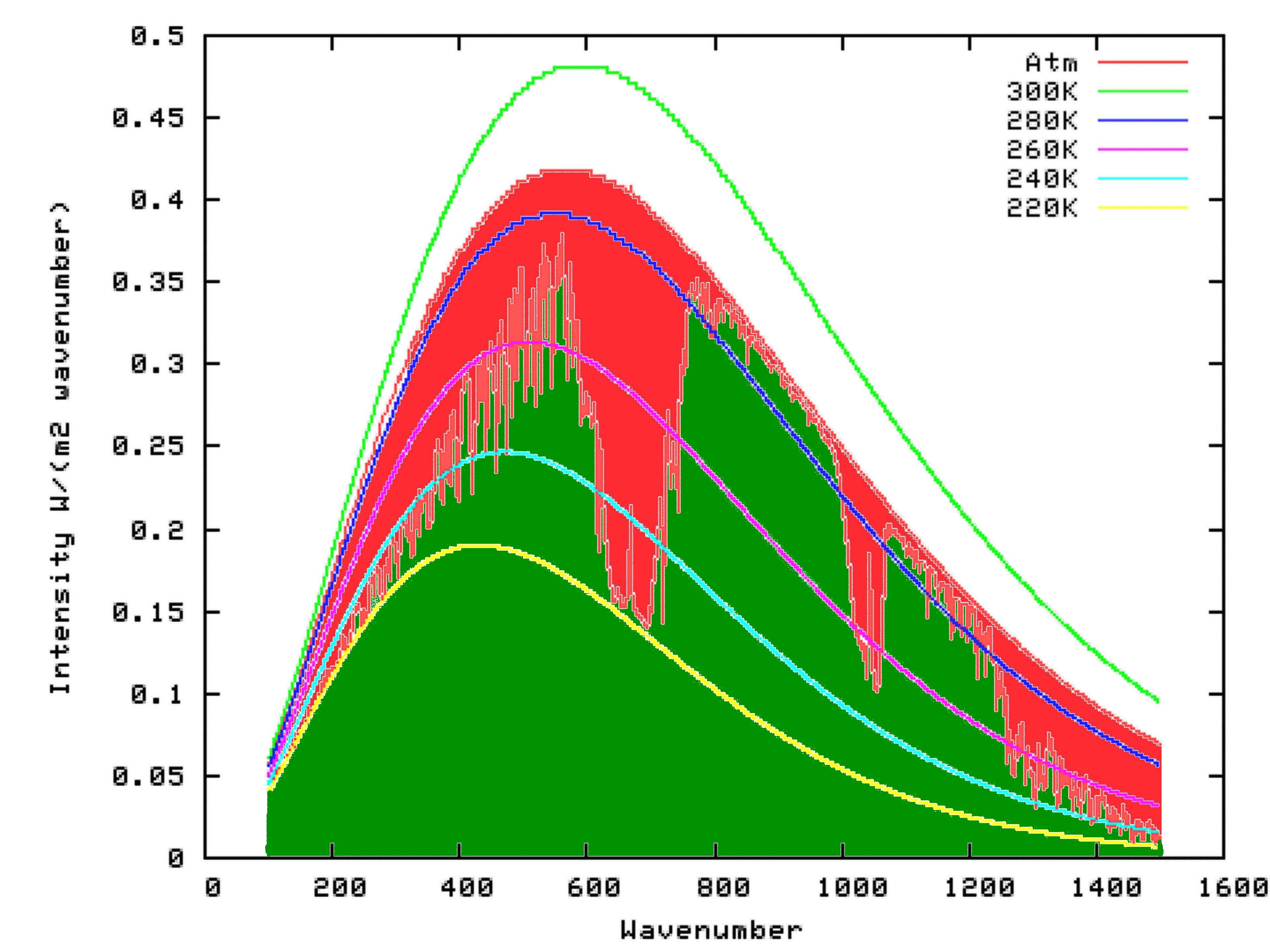
Fortunately for us, scientists have already put this theory into mathematical form, at a very detailed level. We can access this work, free of charge, by using the Modtran Model. The Modtran Model shows the radiation up or down over a column of atmosphere under particular conditions. By changing the conditions, you can explore the predicted effects of those changes on upward or downward radiation at any level of the atmosphere from 0 to 70 kilometers altitude. Setting the altitude to 70 kilometers effectively shows the radiation upward to space from the top of the atmosphere, or downward from space at the top of the atmosphere. Setting the altituded to 0 kilometers effectively shows the radiation upward, or downward at the surface.
Using Modtran, I determined the energy output looking downwards from an altitutude of 70 kilometers using the US Standard Atmosphere (1). The result can be seen on the following graph as the green shaded area. I repeated the model run, but this time with the altitude set at 0 km. The result is shown by the outer curve defining the red area in the graph below. That means that the red area itself, which is the upwards radiation from the surface minus the upward radiation to space, is the reduction in energy radiated to space because of the presence of Infra-Red absorbing molecules in the atmosphere. That is, it is the greenhouse effect.
Settled Science
We have all heard how inaccurate models can be. Therefore the fact that a particular model predicts this difference in radiation only shows what the theory predicts. It does not show what is actually happening.
Scientists are not happy with theories whose only support is a model. So in 1969, Conrath and associates compared the results of model calculations of radiation to space with the actually observed radiation using the IRIS instrument on the Nimbus 3 Satellite. The following graph shows the result of their comparison. The dotted line shows the modelled values, while the solid line shows the observed values:
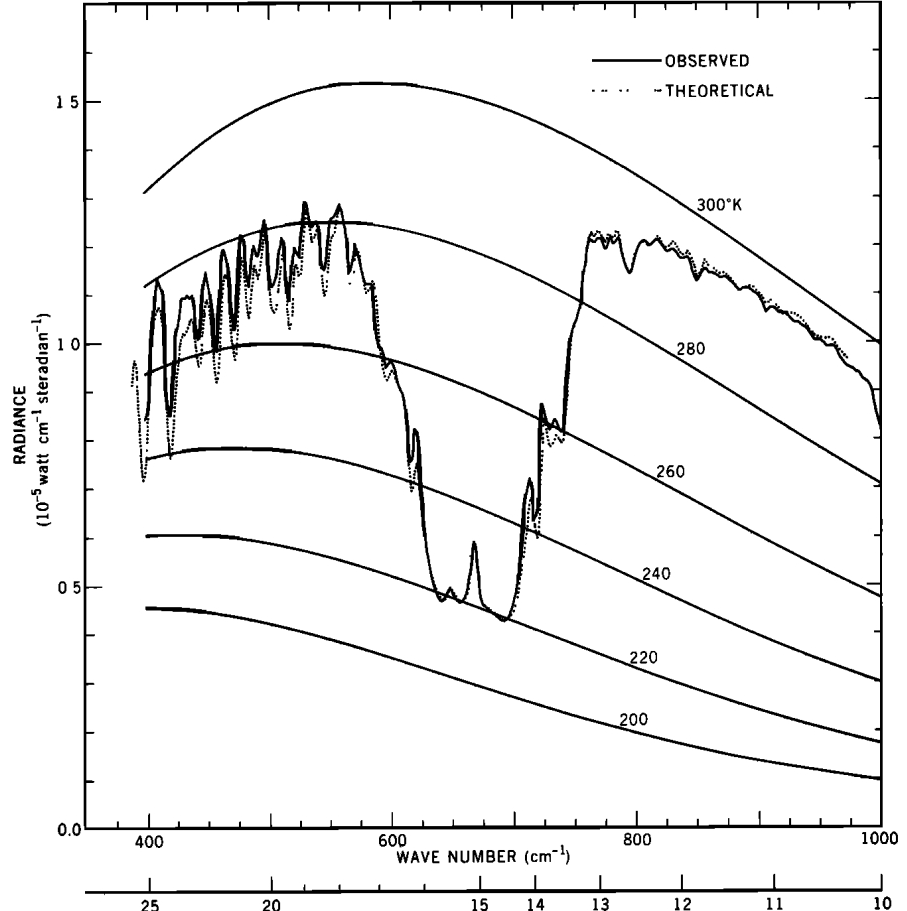
The effect of a particular Infra-Red absorbing molecule, Carbon Dioxide, is clearly visible. With the publication of this data in 1970, the greenhouse effect ceased to be theoretical. It was an observed fact.
Footnote:
(1) Default settings except for adjusting surface temperatures (Ground T offset, c) to approximately match the Earths Global Mean Surface Temperature (about -10 degrees C offset).































 Arguments
Arguments






























In the idealised situation that the climate response to a doubling of atmospheric CO2 consisted of a uniform temperature change only, with no feedbacks operating (but allowing for the enhanced radiative cooling resulting from the temperature increase), the global warming from GCMs would be around 1.2°C (Hansen et al., 1984; Bony et al., 2006).
The issue is the feed backs.- That if you reduce the escape of heat, but do not reduce the incoming heat, things warm up;
- That the atmosphere contains molecules that absorb Infra-Red radiation;
- That radiated energy depends on the temperature of the radiating object; and
- That the atmosphere gets cooler as you get higher, so that the Infra-Red absorbing molecules in the atmosphere radiate less energy to space than they absorb from the surface.
I already understood 1 and 2 and thought that was the whole story, but your 3 and 4 left me a tiny bit confused. Given the system is in equilibrium and has a top-of-atmosphere temperature T0, emitted radiation will be a quantity that can be calculated, say R0. If the result of incoming solar radiation is a surface temperature T1 and the top of atmosphere temperature remains at T0 (ie unable to radiate any more that R0), then the effect will be to change the atmospheric temperature by T1-T0. As the atmosphere is now warmer, the difference between that and the surface is reduced, so the surface stays warmer as well. This is what I understand to be the greenhouse warming effect. What I am missing is why the top of atmosphere does not warm as well, thus radiating more heat into space. Is it because the greenhouse gasses are physically trapping the heat from the surface and preventing it from reaching the TOA? At some point, surely the incoming and outgoing radiation must balance, or we would be living on a cinder. Is this where I am missing something? I can see that the lapse rate means the upper atmosphere is always colder than the lower atmosphere, but given an increase of 1 degree in T1, why does T0 not eventually rise by 1 degree as well, to balance the energy in the system? Or does T0 eventually increase and that becomes the new equilibrium temperature? Sorry if these are dumb questions, but my physics knowledge could be written on the back of a postage stamp and I am really interested in understanding.exceedsis less than the energy entering the system, then the temperature will rise until they balance again. If the energy escapingis less thanexceeds the energy entering system, temperatures will fall until they balance again. Now, if you imagine a series of pipes, with a tap which will be used to force water flowing through the pipe to take a longer or a shorter route. If you switch from the shorter to the longer route, but maintain a constant flow, there will be no build up of pressure, and the reservoir you are draining will not drain any slower. Analogously, if you introduced GHG to the atmosphere so that energy took longer to reach space, but maintained the same temperature at all altitudes so that the amount of energy being emitted to space was not reduce, you would not warm the surface of the planet. Note: Edited to correct an error pointed out by ribwoods @ 6:50 am of March 3rd below. My thanks to him for picking up on my mistype, and my apology for any confusion caused by it.