This post is number 18 in a series about ocean acidification. Other posts: Introduction, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.
Welcome to the last full post in our series about ocean acidification. After this post will be a two part summary. We know CO2 levels have been high in the past. We also know that pH of the ocean has changed before and life survived. What makes this time different? Same answer as for air temperature: rate of change.
There are proxies for past conditions (alkalinity, pH, temperature etc) in the ocean (especially various isotope fractionation processes) but the temporal resolution is not as fine as recent ice core data for atmospheric CO2. However, as we now understand the link between atmospheric and oceanic CO2 we are going to discuss atmospheric CO2.

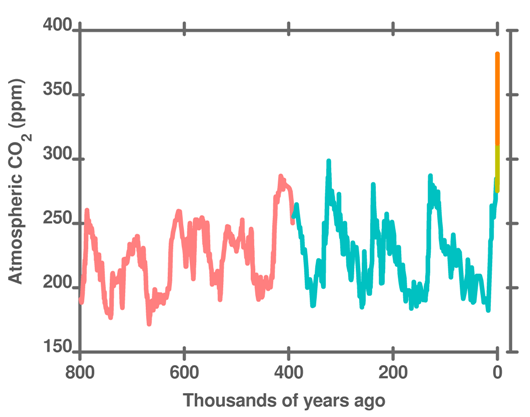
Figure 17. Atmospheric CO2 over the last 8 glacial cycles to modern times. Composite of datasets from EPICA Dome C (800,000-393,000 years), Vostok (393,000-2400 years), Law Dome (1000 – 50 years), and Mauna Loa (50 years – present).
If we go back into deep geological time there have been times when CO2 was higher even than today. But it was a very different planet then. The last time there was a rapid change in ocean pH was the Palaeocene Eocene Thermal maximum (PETM). A post at sceptical science has discussed this already.
1. We are outside normal CO2 levels
The concentration of CO2 in the atmosphere undergoes quite large fluctuations with each ice age to interglacial period. During an ice age, CO2 is about 180 ppm; during an interglacial warm period CO2 increases to about 280 ppm. Atmospheric CO2 was 280 ppm 200 years ago. It is currently (August 2011) 394 ppm. This is well over 100 ppm above the normal recent maximum.
We know that when atmospheric CO2 is high then Henry's law results in the transfer of CO2 from the atmosphere into the ocean. However we also know that the transfer is not instantaneous and that various physical and biological processes we have mentioned in this series of posts mean that there is usually a disequilibrium between atmosphere and ocean. Nevertheless, the oceans will continue to take up CO2. The rate of uptake may slow as the oceans warm and/or circulation patterns change but those changes to ocean temperature and circulation will bring their own problems.
2. Changes are happening faster than normal.
Hollywood notwithstanding, changes in temperature and CO2 at the start or end of ice ages is slow. Each ice age is different, but on average an ice age lasts for a few tens of thousands of years. At the end of an ice age, the 10 deg C temperature change and the 100 ppm CO2 change occurs over 10,000-15,000 years. CO2 is currently increasing at over 2 ppm per year – over 100 times faster than the glacial-interglacial cycle. Henry's law tells us that CO2 in the ocean is changing at almost the same rate. (Variations in the transfer processes discussed in the previous post account for the disequilibrium).
That is, changes in atmospheric CO2 during glacial cycles are slow and occur on the same timescale as the exchange of surface and deepwater. So, CO2 is transported to deep water about as fast as it enters the ocean. However, we are currently adding CO2 to the surface seawater much faster than it can be transported to the deepwater.
Of course, once the CO2 does get into the deepwater then dissolution of CaCO3 sediments will replace some of the lost CO32– but will also increase Total Alkalinity. The ocean carbonate buffer system will not restore the ocean to a preindustrial state. Instead the ocean, and the climate of the Earth as a whole, will converge towards a new stable state. We still don't know precisely what that state will be. It would take another series of 18 posts to describe the current system and several series to describe how we think it will change. OA is not OK.
Before the next two posts of this series (a summary) we want to remind our gentle readers we wrote this series because we had become concerned at misconceptions in wider blogosphere.
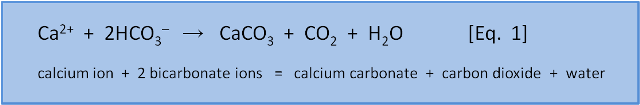
We started with Equation 1 because it is so counterintuitive. Equation 1 is essential if you want to understand ocean acidification. However, despite taking 18 posts and 18,000 words we have only scratched the surface of the chemistry. This is not surprising as the concepts we have introduced do not stand alone. Philately is an enormous endeavour and if we have convinced you that Equation 1 is valid then we are well pleased. If we have failed then it hasn't been for want of barmaiding.
Inevitably we have had to leave some things out and simplified others. For each concept we have explained a dozen more support it and cry out for their 15 minutes. Here is a negligently incomplete list of a further 18 Quite Important concepts – each deserving of at least a post – that we didn't mention of skipped lightly over. There are hundreds more.
δ13C
Ψ
Activity coefficients
Alkalinity
Buffer theory
Burial of CaCO3 sediments
Carbon vs oxygen stoichiometry for combustion of different fossil fuels
Congruent/incongruent dissolution
Conservative ions and charge balance in seawater
Dissolution of CaCO3 above the lysocline
DOM
Fugacity
Inhibition of precipitation
Mass accumulation rate for CaCO3 sediments
Mixed carbonates (when other elements are incorporated)
Net global production of CaCO3
pH scales
Revelle Factor
Gentle reader, the idea that humans can have such an effect on our environment seems almost science fiction. Here is Isaac Asimov - science fiction author and science populariser - in 1989 reminding us that he had been talking about the greenhouse effect for over 20 years. Ocean acidification isn't science fiction. It is happening now.
Written by Doug Mackie, Christina McGraw, and Keith Hunter. This post is number 18 in a series about ocean acidification. Other posts: Introduction, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.
Posted by Doug Mackie on Saturday, 20 August, 2011
 |
The Skeptical Science website by Skeptical Science is licensed under a Creative Commons Attribution 3.0 Unported License. |