You've probably read it before, some aspect of global warming is happening much faster, or sooner than anticipated by the scientific community. Well, this is one of three blog posts that continue in that vein. This time the bad news is corrosive seawater brought about by global warming's evil twin - ocean acidification. Something once considered to be some decades away is already here, and may be killing marine life.
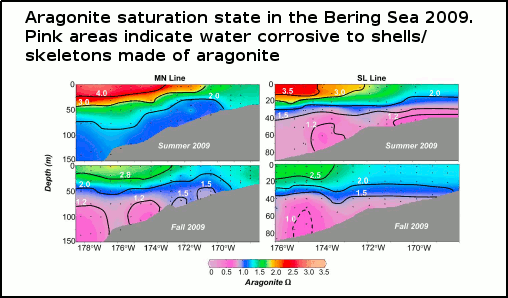
A number of recently published scientific papers (Feeley [2010], Pfister [2011], Mathis [2011a], and Mathis [2011b]) have revealed that, rather than being some far-off future threat, corrosive seawater (from ocean acidification) is making its presence felt, both in the coastal waters of the US/Canadian North Pacific, and the eastern Bering Sea. As well as putting the finishing touches on the struggling oyster farming industry in the US states of Oregon and Washington, ocean acidification is likely to seriously impact the valuable US fishing catch, almost 50% of which (by weight) comes from the Bering Sea. See figure 1.

Figure 1 - Observations of aragonite (shown in colour) and calcite (shown in black with contour lines) saturation states along the transect lines of two oceanographic voyages in the summer and fall of 2009. Pink indicates regions corrosive to aragonite shells/skeletons, and the dashed contour line indicates an area corrosive to calcite shells (a less easily dissolved shell-building material). From Mathis (2011b)
Not only has the burning of fossil fuels increased the level of carbon dioxide in the atmosphere, but almost half of that carbon dioxide has dissolved into the ocean, increased acidity by around 30% (over pre-industrial values) and, crucially for shell-building marine life, it has lowered the concentration of carbonate ions, which they use to build their chalky shells.
Typically, the upper oceans are supersaturated with respect to calcium carbonate (chalk), which simply means there are more than enough carbonate ions in seawater for shell-building. The 3 forms used in shell-building, in descending order of solubility (a measure of how easily they dissolve) being, high-magnesian calcite, aragonite and calcite.
In extreme cases, once the saturation state of calcium carbonate in seawater falls below a value of 1 (undersaturation), seawater frequently becomes corrosive to shells or skeletons made of that particular material. For example, aragonite undersaturation means seawater is corrosive to magnesian-calcite (being more soluble) and aragonite shells, but not calcite, whereas calcite undersaturation means seawater is corrosive to all 3 forms.
Needless to say, this corrosiveness puts the survival of affected marine life into question. Which is not to say that they instantly begin to dissolve, because many adult marine creatures are still able to build shell material in internal chambers sealed off from the surrounding seawater, even as corrosive waters eat away at the outer shell. But they do so at a hugely reduced rate, build weak and deformed shells, and both adult and juvenile survival for many, but not all, marine life, is lower.
The eastern Bering Sea is a vast shallow coastal region off Alaska which is covered by sea ice in the cooler months. Mathis (2011b) carried out analysis of seawater samples collected along two transect lines (paths) from ship-based surveys over spring, summer, and fall in the eastern Bering Sea in 2009. They also collected and analysed cores from sea ice in the area. What they found was that subsurface waters over much of the continental shelf (i.e shallow seas) were seasonally corrosive to aragonite shells, up to 40 metres from the ocean surface. This acidification was worse than previous years, and was due to a combination of factors both natural and man-made.
Firstly, inputs from deep water upwelling, rivers and melting sea ice over warmer months flush low pH (more acidic) water into the eastern Bering Sea, lowering saturation states. But this is offset by the warm season bloom of phytoplankton (microscopic marine-based plants) near the sea surface, which soaks up a lot of carbon dioxide through photosynthesis, and therefore acts to elevate pH (making it less acidic). In effect this drawdown of CO2 into living tissue creates a strong seasonal carbon sink in surface waters, because it the dominant component.
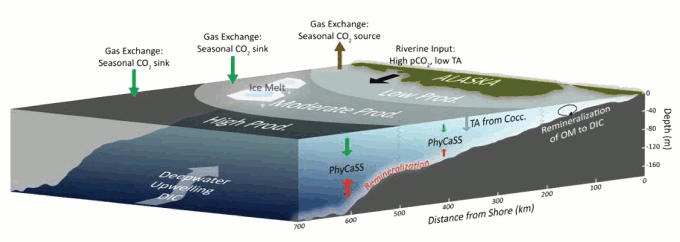
Figure 2 - Generalized description of the processes affecting the carbonate chemistry of the eastern Bering Sea. Biological activity (especially phytoplankton growth and decay) and mixing processes create 3 zones of surface productivity. TA=total alkalinity, and PhyCaSS=phytoplankton-carbonate saturation state. From Mathis (2011b).
The corrosive seawater down near the bottom (figure 1) comes from the phytoplankton (and other organic matter) when they die and sink to the seafloor (phytoplankton have a lifespan often measured in a days). When bacteria begin to break down the bodies of the dead phytoplankton, the carbon dioxide is released, making the bottom water more acidified to such an exent that it becomes corrosive to aragonite, and at times to calcite too. Satellite-based imagery of phytoplankton blooms were seen to match areas of high pH in surface waters, and low pH near the bottom, evidence which supports this long-known transport of carbon to the depths by living things (the biological carbon pump).
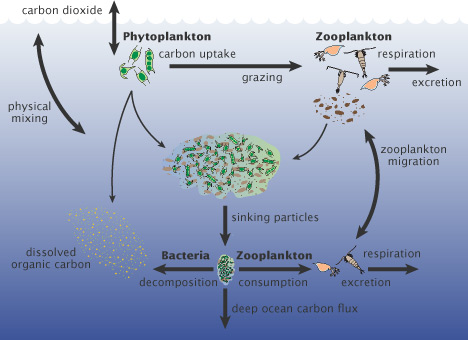
Figure 3 - simple version of the biological carbon pump. Image from the NASA Earth Observatory.
The man-made contribution to change in the eastern Bering Sea is obviously through fossil fuel burning. Although no local measurements were available, the authors made an estimate based upon the north Pacific waters, which feed into the eastern Bering Sea shelf, and where man-made contributions are measured. They estimated that without the human input of CO2, calcite would have been supersaturated all year at all locations, and aragonite supersaturated everywhere, bar one location (the bottom outer shelf) in fall.
In the concluding remarks of their study, the authors state:
"As the anthropogenic CO2 inventories continue to increase in these waters, the saturation states will be further suppressed going forward in time"
and
"........and the Bering Sea will be persistently undersaturated with respect to aragonite likely by mid-century, with broad regions of seasonal calcite undersaturation following later"
So, rather than being a temporary phenomenon lasting for several months at a time, as it is now, human fossil fuel CO2 will lead to both expansion of, and longer periods of corrosive waters, until the entire water column is persistently corrosive.
What does this all mean for the lucrative fishing industry based in the Bering Sea? It's too early to say for certain. The dire threat posed by ocean acidification, rather embarrassingly, has only crept into mainstream scientific awareness in the last decade or so. Therefore few long-term records or baseline studies exist, and marine scientists are, more-or-less, scrambling to quickly find out the implications.
Regardless, there are some studies which hint at a bad outlook for the Bering Sea. A keystone species (major player) of the Bering Sea foodweb are pteropods, tiny swimming sea snails, or "sea butterflies," which feed upon phytoplankton and zooplankton. These are a major food source for the commercially important pollock and salmon fisheries, especially in their juvenile stages.
Feeley (2004) found that the shell of these pteropods dissolved when exposed to seawater undersaturated in aragonite. Comeau (2010) also witnessed corrosive waters dissolving the shell of a Mediterranean pteropod, and found that it was even able to survive (in the protected study environment) without a shell, although Comeau (2011) point out that it's highly unlikely that such shell-less 'sea butterflies' would survive in a natural environment. But perhaps of greater relevance is Lischka (2011), whose authors observed that not only did aragonite undersaturation dissolve the shells of a common Bering Sea pteropod, but it also caused higher rates of mortality, as did elevated temperatures (which are occurring in the Bering Sea, in response to global warming).
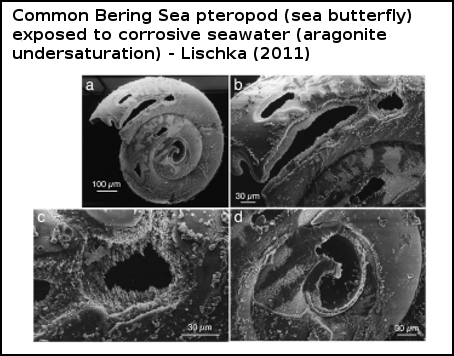
Figure 4 - scanning electron micograph of pteropod Limacina helicina shell reared at 3°C and in aragonite undersaturated seawater. Magnification and scale bars are shown. The shell is severely corroded by aragonite undersaturation. From Lischka (2011).
Therefore one of the 'foundation blocks' of the Bering Sea ecosystem might be under severe threat with the projected expansion and intensification of corrosive waters and ocean warming, and the depletion of such a key food source will very likely reduce fish populations.
So to summarize the key points:
Next - Part 2: Corrosive water in the US/Canadian North Pacific: is it killing oysters?
Related SkS posts: OA not OK series, Acidification:Oceans past, present & yet to come, Ocean acidification: Some Winners, Many Losers, Ocean acidification: global warming's evil twin and Great Barrier Reef Part 3: Acidification, Warming, and Past Coral Survival.
Posted by Rob Painting on Wednesday, 14 December, 2011
 |
The Skeptical Science website by Skeptical Science is licensed under a Creative Commons Attribution 3.0 Unported License. |