This post is number #13 in a series about ocean acidification. Other posts: Introduction, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.
Welcome to the 13th post in our series about ocean acidification. In post 3, we said that there are several types of calcium carbonate. In this post, we describe the types of calcium carbonate used in by marine organisms to make their shells.
Shelled fauna first show up in fossil records for the early Cambrian period – about 540 million years ago, some 60 million years after the evolution of multicellular organisms.
Why calcium carbonate for shells? If truth be told, it isn't always calcium carbonate; other bio-minerals are also used. Radiolaria and diatoms, are single-celled photosynthetic algae that have skeletons made of silica, as do most sponges. Vertebrates, on the other hand, favour various phosphates.
But calcium carbonate is the most common marine biomineral. Why? In a single sentence, calcium carbonate is hard, (mostly) insoluble, and cheap. Actually, there are several forms of calcium carbonate and we may as well get the distinctions clear now.
Biological calcium carbonate comes in two main forms, or polymorphs poly = many, morph = form): aragonite and calcite. A third form, vaterite, is quite soluble and is rare in biology.
Aragonite is found, for example, in thecosomata pteropods (small free swimming sea snails) and corals. Calcite is found, for example, in coccolithophores and foraminifera. Molluscs can use either or both polymorphs.

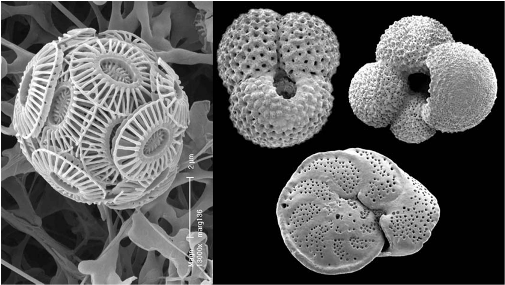
Figure 7. (L to R). Emiliana huxleyi a common coccolithophore about 5 microns across, (image ORNL ), fossil foraminifera from New Zealand about 500 microns across, (image Te Ara).
Without going into great detail (fascinating though it is) we will say that the polymorphs differ in the way the molecules stack when they form crystals. As an analogy, think of stacking balls in a box. Figure 8 shows two of the several ways to stack spheres. The left panels show 2 ways to lay the bottom layer and the right panels show 2 ways to lay the top layer. In the top row of boxes, each ball not on an edge touches 4 neighbours in a given layer (plus 4 in each layer above and below). In the second row of boxes each ball not on an edge touches 6 neighbours in a given layer (plus 3 in each layer above and below). That is balls in both ways of stacking have the same number of neighbours – though the neighbourhood makeup is different.
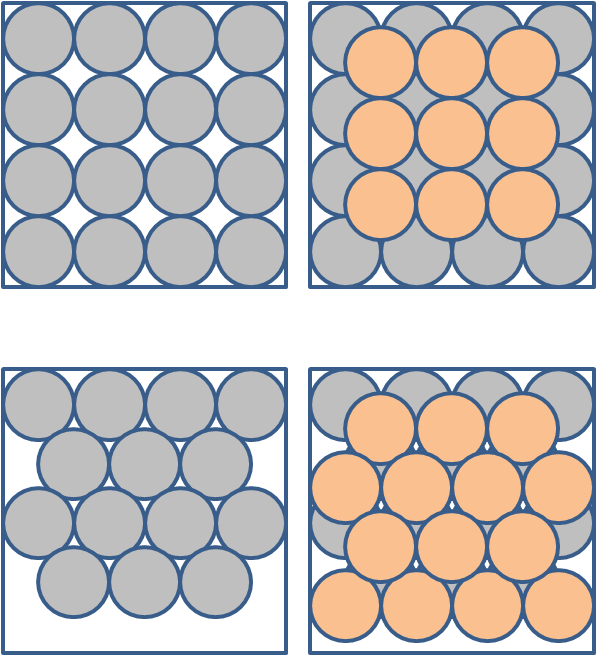
Figure 8. Some ways to pack spheres. Left panels give two ways to lay the first layer. Right gives second layer - laid as for first layer. These are NOT representations of aragonite and calcite.
Note that these figures do NOT represent aragonite or calcite. It would take a lot of subtle technical terms to adequately explain their actual crystal structure, but the basic principles exemplified in the figures holds. You can see that the spheres in the bottom boxes are packed more tightly than the spheres in the top boxes. This sort of packing difference explains why aragonite is denser (2.95 grams per cubic centimetre) than calcite (2.71). The packing arrangement (including things like the length of bonds between molecules and the makeup of the neighbours) also controls the attributes like solubility. In the case of CaCO3, the polymorph aragonite is always more soluble than calcite.
![]()
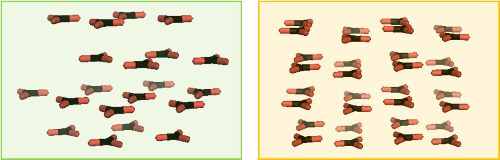
Figure 9. Crystal structures of carbonate molecules in calcite (L) and aragonite (R). Carbonate groups are shown as 'Y' shape sticks. Black = carbon, red = oxygen. The differences are primarily in the way the CO32– molecules align or are staggered so calcium atoms omitted for clarity.
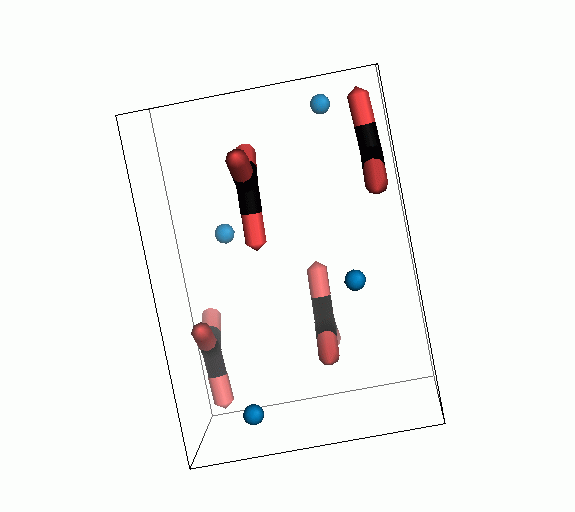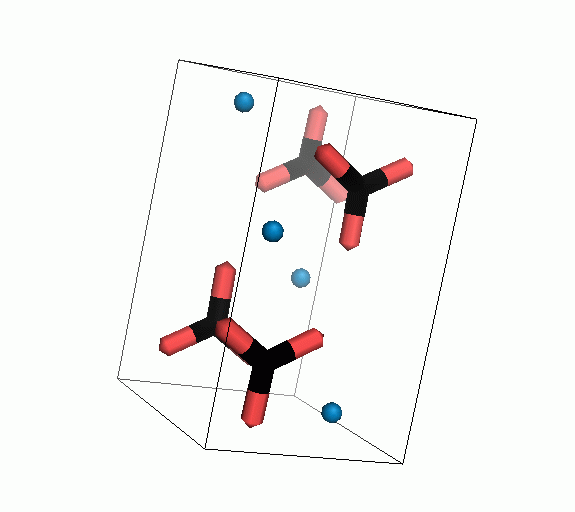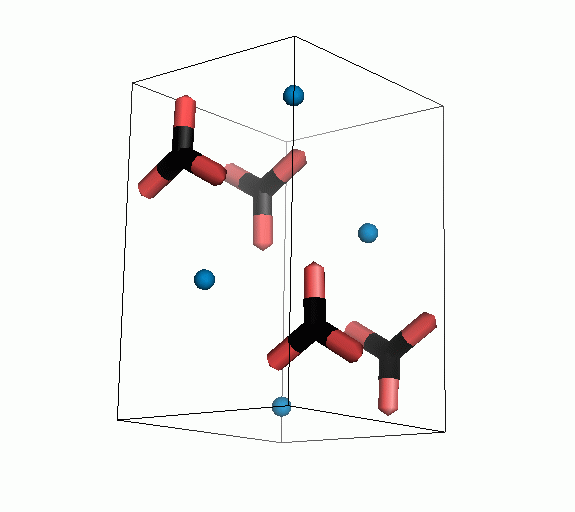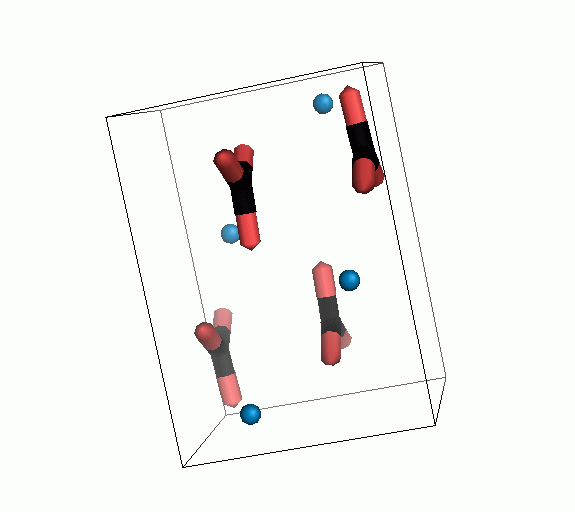
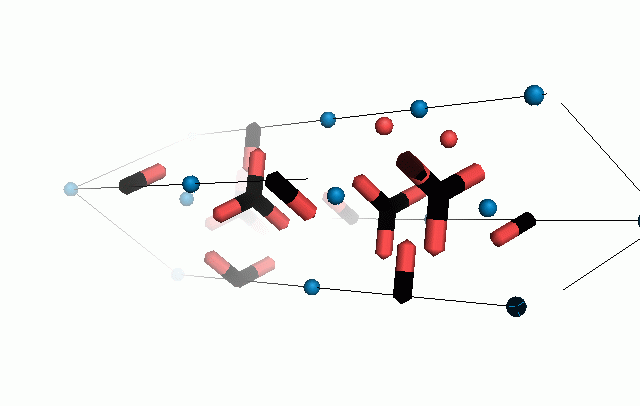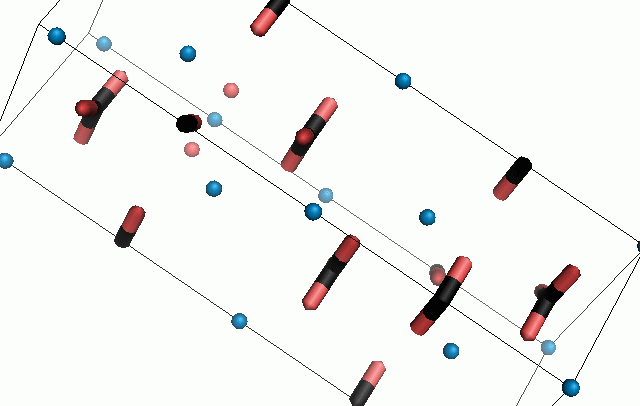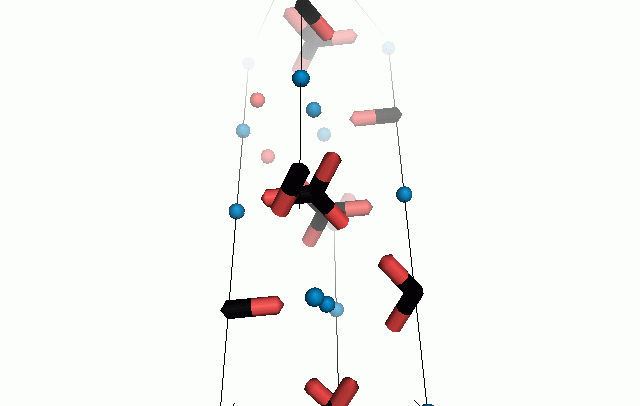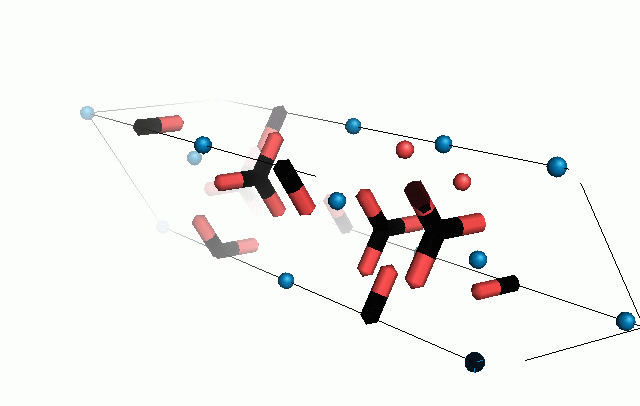
Figure 10. Animations of the unit cells for crystal structures of aragonite (top) and calcite (bottom). Carbonates shown as 'Y' shape sticks. Black = carbon, red = oxygen, blue = calcium. The black boxes outline the unit cell
The unit cell is the repeating unit of the crystal. This is the same principle as wallpaper pattern groups. A simple repeating unit is used to build up the pattern. Sometimes a simple pattern is reproduced within a short space, other times it takes much longer before the pattern repeats. It turns out that the calcite unit cell is 3x longer than the aragonite unit cell and that the calcite unit cell contains partial CO32- (hence the funny looking non Y shapes at the edges).
Don't think that the molecules are mostly empty space. The 'stick' form structures are given because they highlight spatial relationships between the CO32- molecules. Space-fill representations show the electrical sphere of influence of each atom (Figure 11) but obscure the molecule to molecule positioning.
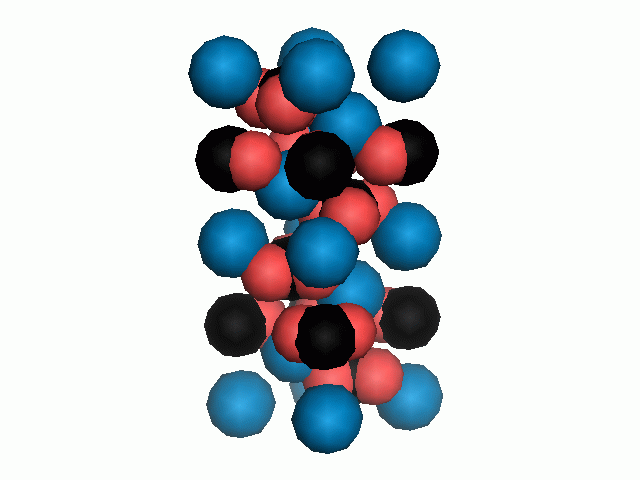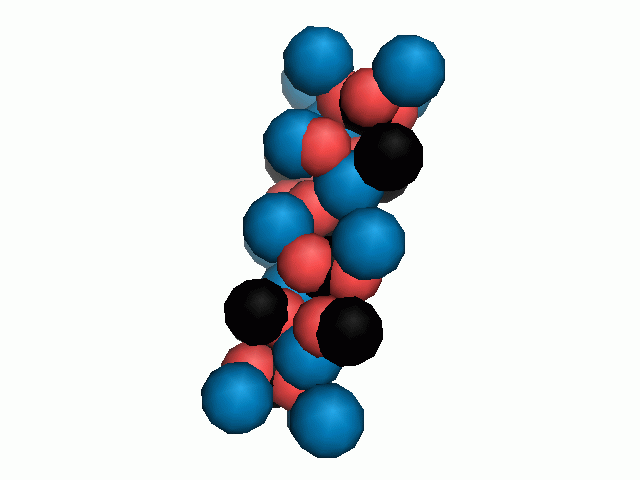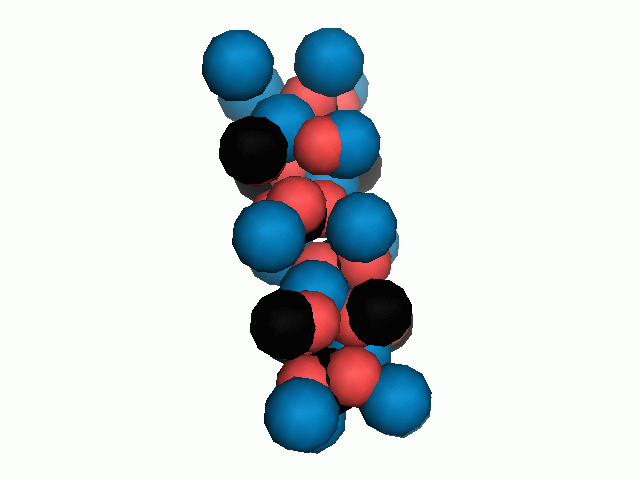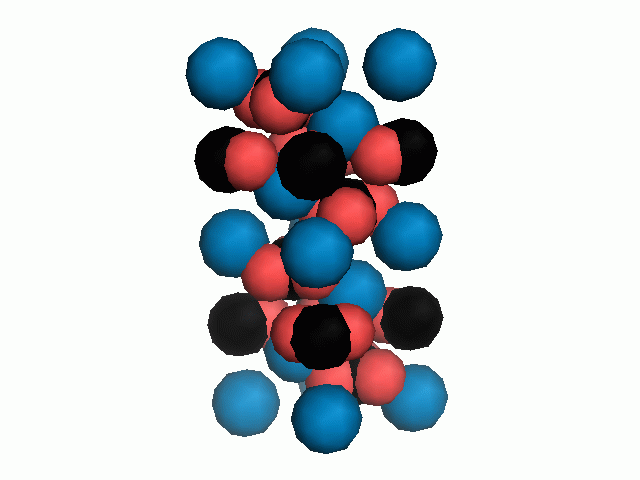
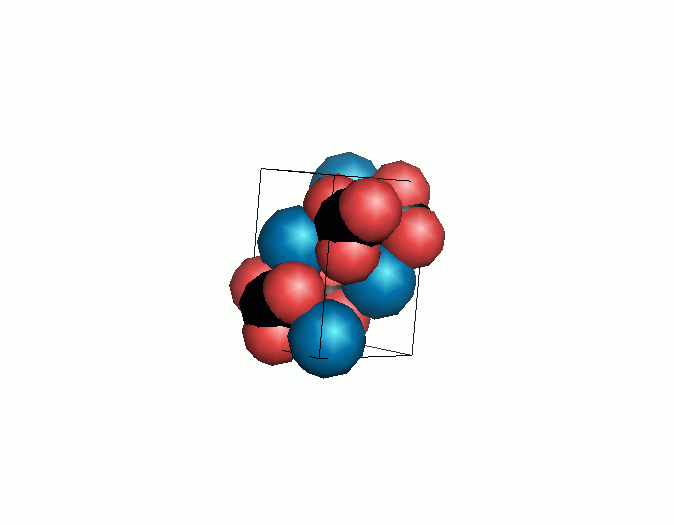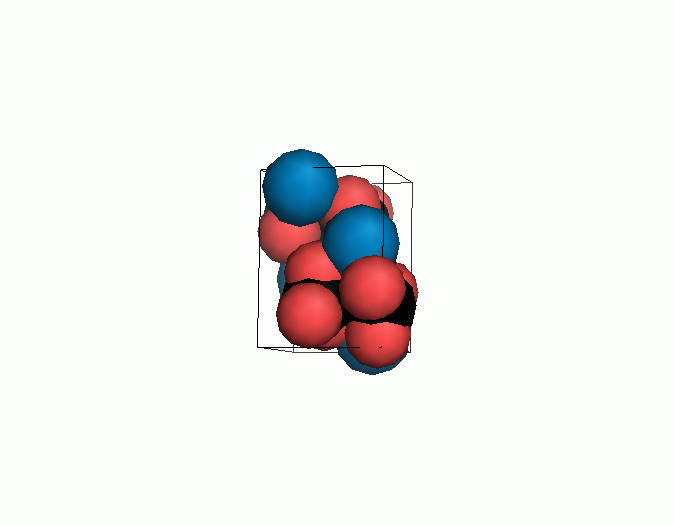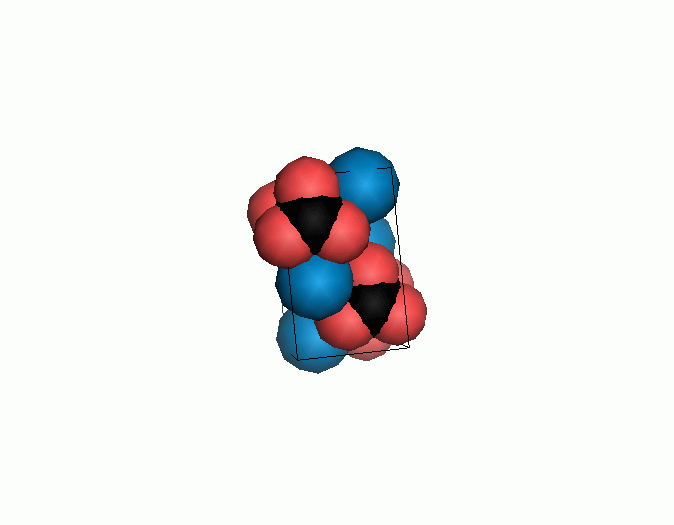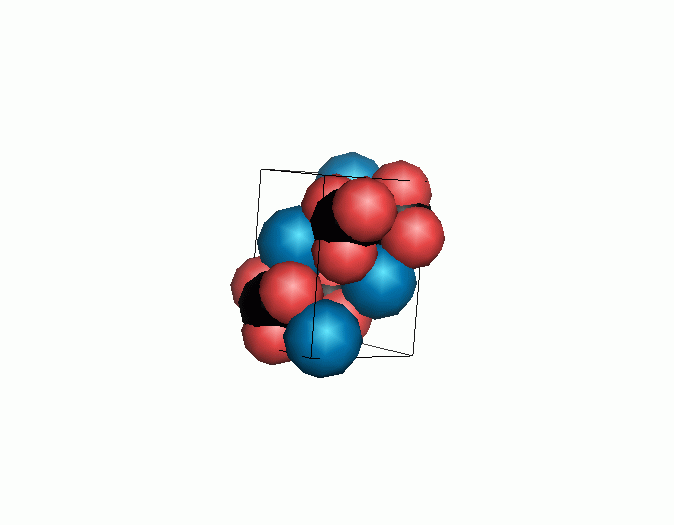
Figure 11. Space filling sphere representations of unit cell for calcite (left) and aragonite (right).
How is the stability of calcium carbonate shells affected by the particular polymorph? In post 2 we mentioned that lower energy reaction products are favoured by thermodynamics. For 2 or more polymorphs we can illustrate this graphically. The thick blue line in Figure 12 shows the relative energy of calcium carbonate with respect to packing conditions. The two dips in the line correspond to two states where a compound is stable. Imagine placing a ball in each dip - aragonite (pink ball) and calcite (green ball). The green ball (calcite) is well and truly sunk in its pocket and is thus stable. Aragonite is formally described as metastable: the pink ball mostly stays put, but a gentle push could get it roll down to calcite.
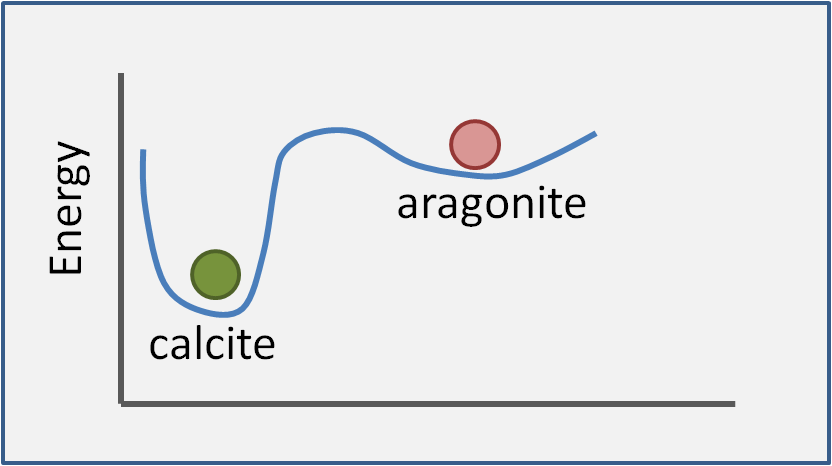
Figure 12. Stability of polymorphs of calcium carbonate. The schematic illustrates the generic concept of polymorphs but is not accurate for aragonite vs. calcite. For one thing, the relative stabilities depend on temperature, pH etc.
Ever since Rutherford chemists have had a funny idea about what 'gentle' is and in this case a 'gentle push' means heating to a couple of hundred of degrees and/or sitting around for a few million years. So in the real world, under most conditions, aragonite shells are as stable as calcite shells. We will, of course return, to 'most conditions' and what the choice of polymorph means for organisms in a later post.
Rutherford was one of ours (a kiwi) but this post couldn't have been written without an Australian who was 25 years when he got his prize, he'd be a 121 now if he hadn't died.
In the next post we discuss the main processes that shift and transform carbon in the ocean: photosynthesis and respiration.
Written by Doug Mackie, Christina McGraw , and Keith Hunter . This post is number 13 in a series about ocean acidification. Other posts: Introduction , 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.
Posted by Doug Mackie on Friday, 5 August, 2011
 |
The Skeptical Science website by Skeptical Science is licensed under a Creative Commons Attribution 3.0 Unported License. |