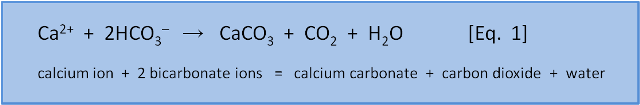
This is part 1 of 2 summarising out series about ocean acidification. The posts: Introduction , 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.
During July and August we posted an 18 part series to introduce the basics of ocean acidification chemistry. In this post we summarise posts 1-10. We have distilled each post to the bare minimum (100 words average). Naturally a lot has been lost; please refer to the original posts.
Part 1: OA not OK
Ocean acidification is the process of ocean pH decreasing (i.e. becoming more acidic) due to absorption of fossil fuel CO2 from the atmosphere. Another effect of ocean acidification is to reduce the amount of carbonate that is available to marine organisms, such as shellfish, for making their calcium carbonate shells.
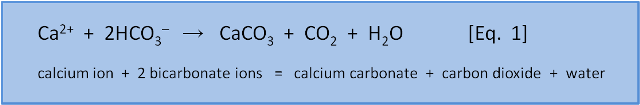
![]()
Part 2: Thermodynamic duo
Equation 1 is spontaneous in the surface ocean and shellfish etc extract bicarbonate ions from seawater to make their skeletons and shells. However, those conditions can be changed so that the reverse reaction happens, causing the calcium carbonate to dissolve via Equation 4. This is what takes place when limestone rocks are weathered by the action of rain and air.

Part 3: Wherever I lay my shell, that's my home
A solution that contains the maximum possible dissolved amount of a salt (like CaCO3) is saturated. If you add more, then the salt will precipitate until the excess has been removed. A solution can be supersaturated and contain more ions that can combine to form a salt than is 'theoretically' possible. Surface seawater is supersaturated with CaCO3. This means it takes very little effort to precipitate CaCO3 from seawater.
Part 4: The f-word: pH
Average ocean pH has decreased by 0.11 pH units (from 8.25 to 8.14) since the industrial revolution and will decrease a further 0.3 units to 7.8 by 2100. A difference of 0.11 pH units corresponds to a 29% increase in the concentration of H3O+. A difference of 0.4 pH units corresponds to a 150% increase in H3O+.

Part 5: Reservoir dogs
Equations 7-9 describe reactions of the inorganic carbon in seawater. The balances between these equations mean 91% of carbon is in the form of bicarbonate (HCO3–), 8% as carbonate (CO32–), and less than 1% is found as CO2 and H2CO3.
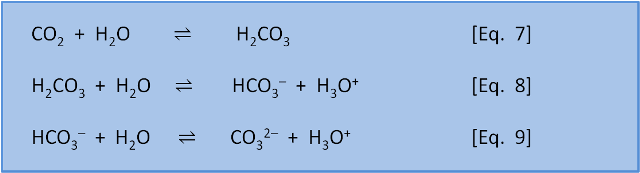
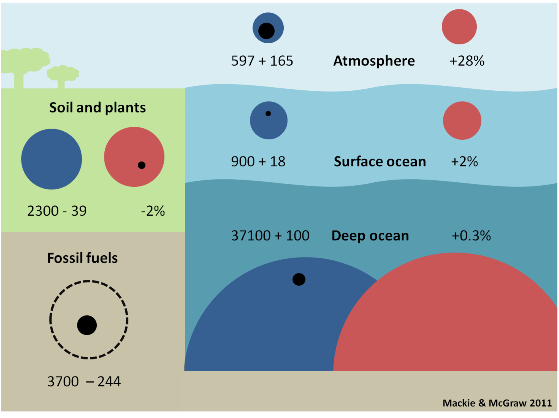
Figure 2. Carbon reservoirs as preindustrial size (blue circles) and modern size (red circles) with change since the industrial revolution (black circles). Numbers give preindustrial size with change (+ or –) and also express the change as a %. Size in giga tons of carbon = Gt C. (1 Gt = 1,000 million tons, i.e. billion tons).
Part 6: Always take the weathering
Weathering occurs when rainwater reacts with carbonate rocks. Rainwater is in equilibrium with atmospheric CO2, so carbonic acid is formed via Eq. 7. This leads to mildly acidic rainwater (pH 5.7). Weathering consumes CO2 and means that river water contains a lot of bicarbonate. The amount of bicarbonate added to the ocean by rivers is equal to the amount of CO2 consumed and is sufficient to remove all CO2 from the atmosphere in 3500 years. Plainly this hasn't happened in the past. Something is returning CO2 to the atmosphere. That something is Eq. 1 for calcification.

![]()
Part 7: Le Chatelier not good enough
Many readers may be familiar Le Chatelier’s Principle as a way to predict how an equilibrium reaction responds when it is disturbed. However, it only applies to single-step chemical reactions and can't be applied to coupled or sequential reactions. For equation 9 Le Chatelier predicts that if H3O+ (i.e. acid) is added then it will force the reaction to the left side, producing bicarbonate. However, for equation 8 that is occurring at the same time, Le Chatelier predicts that if acid is added then this should force the reaction to the left side, consuming bicarbonate.
Part 8: 170 to 1
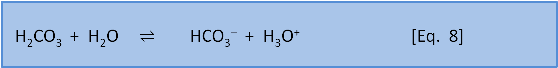
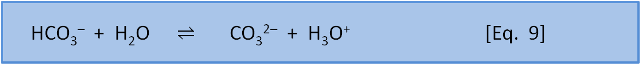
For Eq. 8, in seawater at the preindustrial ocean pH of 8.25, the equilibrium ratio of the left side, carbonic acid, to the right side, bicarbonate, is about 1:170. For Eq. 9 the ratio of the left side, bicarbonate, to the right side, carbonate, is about 9:1.
Equation 8 says that if we do add a little acid (H3O+), then 170 parts of the acid stay as acid (H3O+) and only 1 part reacts to form carbonic acid and water. But equation 9 tells us that if acid is added then 1 part of the acid remains as H3O+ but 9 parts of the acid, and therefore some of the carbonate, are consumed to produce bicarbonate. At typical seawater pH, the response of the system to the addition of acid is dominated by the consumption of carbonate shown in Equation 9.
Adding acid to the seawater-CO2 system massively changes the proportion of carbonate but hardly changes the proportion of bicarbonate since most of the carbon is already in the form of bicarbonate. Since preindustrial times the concentration of carbonate has changed by –25% but the concentration of bicarbonate HCO3– has only changed by 3%.
![]()
Part 9: Henry the 8th I was*
Henry's Law of gas solubility says that for a liquid in equilibrium with the gas above it, the amount of gas absorbed in the liquid is directly proportional to the partial pressure of the gas. Calculations are complicated because of biological activity and because absorbed CO2 forms carbonic acid. However the concentration of CO2 in the atmosphere and the oceans are directly related.
Part 10: Is the ocean blowing bubbles?
Four observations show the extra CO2 in the atmosphere has come from the combustion of fossil fuels and not from outgassing of CO2 from the ocean:
1. Oxygen decrease. Atmospheric oxygen (O2) is going down by the same amount that atmospheric CO2 is going up. See AR4 Figure 2-3.
2. Isotope ratios. Observations show that the isotope ratios of carbon in the atmosphere are changing due to an influx of CO2 depleted in 13C – as occurs in fossil fuels.
3. Not enough warming. Warm water can hold less CO2 than cold water. To explain the 100 ppm of additional CO2 added to the atmosphere since preindustrial times, the average temperature rise of the surface ocean needs to be about 10o C, much larger than has occurred.
4. Known fossil fuel CO2 emissions. Any alternative explanation for the source of the CO2 in the atmosphere must explain where the 30 billion tonnes of CO2 released yearly by fossil fuel burning goes. However, the amount of released fossil fuel CO2 is more than the amount of extra CO2 that is currently in the atmosphere.
Next post: Summary parts 11-18
Written by Doug Mackie, Christina McGraw, and Keith Hunter. Other posts: Introduction, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.
Posted by Doug Mackie on Thursday, 25 August, 2011
 |
The Skeptical Science website by Skeptical Science is licensed under a Creative Commons Attribution 3.0 Unported License. |