How do we know more CO2 is causing warming?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
An enhanced greenhouse effect from CO2 has been confirmed by multiple lines of empirical evidence. |
|||||||
Climate Myth...
Increasing CO2 has little to no effect
"While major green house gas H2O substantially warms the Earth, minor green house gases such as CO2 have little effect.... The 6-fold increase in hydrocarbon use since 1940 has had no noticeable effect on atmospheric temperature ... " (Environmental Effects of Increased Atmospheric Carbon Dioxide)
At-a-glance
To make a statement like, "minor greenhouse gases such as CO2 have little effect", is to ignore 160 years of science history. So let's look at who figured out the heat-trapping properties of carbon dioxide and when.
Experiments involving various gas mixtures had demonstrated the heat-trapping properties of water vapour, CO2 and methane in the 1850s. But those effects were yet to be quantified - there were no meaningful numbers. It was to be another 40 years before that happened.
Swedish scientist Svante Arrhenius (1859-1927) was the person who crunched the numbers. The results were presented in a remarkable paper, "On the Influence of Carbonic Acid in the Air upon the Temperature of the Ground", in 1896.
The many calculations in the 1896 paper include estimates of the amounts of CO2 increase or decrease required to drive the climate into a different state. One example used was the Hothouse climate of the Cenozoic, around 50 million years ago. Another was the glaciations of the last few hundred millennia.
To get a temperature rise of 8-9°C in the Arctic, Arrhenius calculated that CO2 levels would have to increase by 2.5 to 3 times 1890s levels. To lower the temperature 4–5°C to return to glacial conditions, he calculated a drop in CO2 was needed of 0.62-0.55 times 1890s levels.
We know CO2 levels in the 1890s from ice-core data. They were around 295 ppm. Let's do the sums. A reduction factor of 0.55 to 0.62 on 295 ppm gives 162.2-183.9 ppm. Modern ice-core measurements representing the past 800,000 years show that in glacial periods, CO2 levels fell to 170-180 ppm.
What we now know due to additional research since 1896 when Arrhenius worked on this, is that CO2 was an essential 'amplifying feedback'. That means changes triggered by long term, cyclic variations in Earth's orbit cause warming or cooling and CO2 release or entrapment in turn. Those changes in CO2 levels affected the strength of Earth's greenhouse effect. Changes in the strength of the greenhouse effect then completed the job of pushing conditions from interglacial to glacial - or vice-versa.
Arrhenius also made an important point regarding water vapour: "From observations made during balloon voyages, we know also that the distribution of the aqueous vapour may be very irregular, and different from the ideal mean distribution." This statement holds true today: water vapour is a greenhouse gas but because water exists in gas, liquid and solid forms in the atmosphere, it is continually cycling in and out of the air. It is distributed in a highly uneven fashion and is uncommon in the upper atmosphere. That's where it differs from CO2.
Once CO2 is up there, it's up there for a long time. As a consequence it has a pretty even distribution: 'well-mixed' is the term. As Arrhenius quantified all that time ago, once it's up there it constantly absorbs and re-radiates heat in all directions. That's why dumping 44 billion tons of it into our atmosphere in just one year (2019 - IPCC Sixth Assessment Report 2022) is a really bad idea.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
Good scientific theories are said to have ‘predictive power’. In other words, armed only with a theory, we should be able to make predictions about a subject. If the theory’s any good, the predictions will come true.
Here’s an example: when the Periodic Table of the chemical elements was proposed in 1869, many elements were yet to be discovered. Using the theory behind the Periodic Table, the Russian chemist Dmitri Mendeleev was able to predict the properties of germanium, gallium and scandium prior to their discovery in 1886, 1875 and 1879 respectively. His predictions were found to be correct.
The effect on Earth's greenhouse effect of adding man-made CO2 is predicted in the theory of greenhouse gases. This theory was first proposed by Swedish scientist Svante Arrhenius in 1896, based on earlier work by Fourier, Foote and Tyndall. Many scientists have refined the theory since Arrhenius published his work in 1896. Nearly all have reached the same conclusion: if we increase the amount of greenhouse gases in the atmosphere, the Earth will warm up.
Where there is less agreement is with respect to the exact amount of warming. This issue is called 'climate sensitivity', the amount the temperatures will increase if CO2 is doubled from pre-industrial levels. Climate models have predicted the least temperature rise would be on average 1.65°C (2.97°F) , but upper estimates vary a lot, averaging 5.2°C (9.36°F). Current best estimates are for a rise of around 3°C (5.4°F), with a likely maximum of 4.5°C (8.1°F). A key reason for this range of outcomes is because of the large number of potential climate feedbacks and their variable interactions with one another. Put simply, some are much better understood than others.
What Goes Down…
The greenhouse effect works like this: Energy arrives from the sun in the form of visible light and ultraviolet radiation. The Earth then emits some of this energy as infrared radiation. Greenhouse gases in the atmosphere 'capture' some of this heat, then re-emit it in all directions - including back to the Earth's surface.
Through this process, CO2 and other greenhouse gases keep the Earth’s surface 33°Celsius (59.4°F) warmer than it would be without them. We have added 42% more CO2, and temperatures have gone up. There should be some evidence that links CO2 to the temperature rise.
So far, the average global temperature has gone up by more than 1 degrees C (1.9°F):
"According to an ongoing temperature analysis led by scientists at NASA’s Goddard Institute for Space Studies (GISS), the average global temperature on Earth has increased by at least 1.1° Celsius (1.9° Fahrenheit) since 1880. The majority of the warming has occurred since 1975, at a rate of roughly 0.15 to 0.20°C per decade."
The temperatures are going up, just like the theory predicted. But where’s the connection with CO2, or other greenhouse gases like methane, ozone or nitrous oxide?
The connection can be found in the spectrum of greenhouse radiation. Using high-resolution FTIR spectroscopy, we can measure the exact wavelengths of long-wave (infrared) radiation reaching the ground.
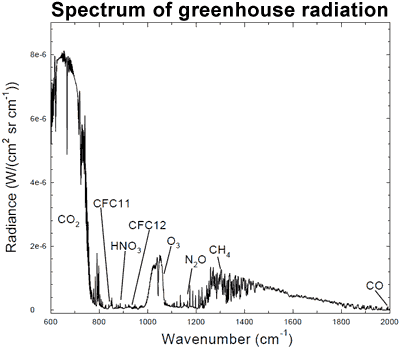
Figure 1: Spectrum of the greenhouse radiation measured at the surface. Greenhouse effect from water vapour is filtered out, showing the contributions of other greenhouse gases (Evans 2006).
Sure enough, we can see that CO2 is adding considerable warming, along with ozone (O3) and methane (CH4). This is called surface radiative forcing, and the measurements are part of the empirical evidence that CO2 is causing the warming.
...Must Go Up
How long has CO2 been contributing to increased warming? According to NASA, “Two-thirds of the warming has occurred since 1975”. Is there a reliable way to identify CO2’s influence on temperatures over that period?
There is: we can measure the wavelengths of long-wave radiation leaving the Earth (upward radiation). Satellites have recorded the Earth's outgoing radiation. We can examine the spectrum of upward long-wave radiation in 1970 and 1997 to see if there are changes.
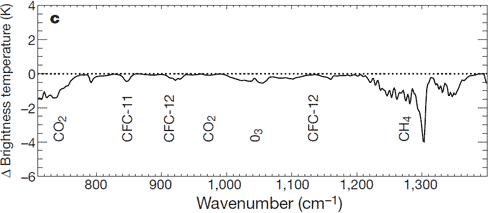
Figure 2: Change in spectrum from 1970 to 1996 due to trace gases. 'Brightness temperature' indicates equivalent blackbody temperature (Harries et al. 2001).
This time, we see that during the period when temperatures increased the most, emissions of upward radiation have decreased through radiative trapping at exactly the same wavenumbers as they increased for downward radiation. The same greenhouse gases are identified: CO2, methane, ozone and so on.
The Empirical Evidence
As temperatures started to rise, scientists became more and more interested in the cause. Many theories were proposed. All save one have fallen by the wayside, discarded for lack of evidence. One theory alone has stood the test of time, strengthened by experiments.
We have known CO2 absorbs and re-emits longwave radiation, since the days of Foote, Tyndall and Arrhenius in the 19th Century. The theory of greenhouse gases predicts that if we increase the proportion of greenhouse gases, more warming will occur.
Scientists have measured the influence of CO2 on both incoming solar energy and outgoing long-wave radiation. Less longwave radiation is escaping to space at the specific wavelengths of greenhouse gases. Increased longwave radiation is measured at the surface of the Earth at the same wavelengths.
Last updated on 16 July 2023 by John Mason. View Archives































 Arguments
Arguments






































Rudmop... So, just to be clear, you are aware that observations contradict your assertions. Right?
Rudmop: If you have not already done so, I highly recommend that you peruse The Science of Doom website. It is full of equations about key components of the Earth's climate system.
The second piece of block text by Rudmop @238 is a response to my response to his first posting of his ideas on another thread. Briefly, my criticisms were that:
1) "The strength of the greenhouse effect of a given gas is a direct function of the difference in power radiated to space by that gas and the power radiated by the surface, and intercepted from going to space by that gas. As the power radiated to space is an inverse function of the temperature of the gas at the mean altitude of radiation to space, the vertical distribution of the concentration of relevant gases is a fundamental property without which no valid determination of relative greenhouse effect of different gases can be made."
2) "The energy trapping capability of each molecule is not simply a function of the sum of the energies at the absorption peaks in the spectra. It is also a function of the relative energy radiated at those wavelengths from the surface"
3) "You have not explained, and nor can I see what relevance rates of diffusion have on the result. In particular, concentration levels of CO2 (in particular) and to a lesser extent H2O are fairly stable so that rates of change in the concentration in still air (diffusion) have no bearing on spatial patterns of concentration, which you do not allow for in your equation in any event."
I note that Rudmop has changed his formula to take into account my second point.
He rejects my first point saying:
What Rudmop neglects in this response is that emissivity equals absorptivity. It follows that if a given thickness of atmosphere has more radiation at a given wavelength impacting upon it then it will radiate due to its temperature, it will absorb more radiation than it emits. As the CO2 in the upper troposphere is colder than that in the lower troposphere, or at the surface, it will absorb more radiation than it emits, and therefore the upward IR radiation from that layer will be less than the upward IR radiation entering that layer.
The notion that a CO2 molecule "... cannot hold on to any trapped energy for an indefinite amount of time", while accurate, is irrelevant. Even at 85 km altitude (US standard atmosphere), an excited CO2 (or H2O) molecule will, on average, experience 380,000 (a million) collisions before it would typically have spontaneiously returned to a base energy state by emitting a photon. Within the troposphere the figure is closer to 5 billion collisions. Therefore absorbed radiation is rapidly transmitted to the rest of the atmosphere as heat, and stored by the whole atmospheric layer. The emissions from that layer, in turn, are almost exclusively from CO2 (or other greenhouse gases) that have entered an excited state due to collissions from with other molecules. That is why the emission fits the profile of thermal radiation (within the radiating wavelengths). And because the radiation is thermal, it is controlled by the temperature of the layer, not the rate of absorption of photons from lower layers in the atmosphere.
With regard to my third point, Rudmop now clarrifies by saying:
In fact, the relevant ratio here is the rate of natural reemission molecules in an excited state to the rate of collision. As noted above, that does not rise above about 1 in a 380,000 below the thermosphere, either for CO2 or H2O. Allowing that CO2 radiates before collisions 2.5 times as frequently as H2O, that only raises its non-thermal radiation to 0.0003% of the total (compared to 0.0001% for H2O) even in the upper mesosphere. In short, the difference is negligible for all practical purposes.
Rudmop - I'm afraid your arguments, no matter how elegant, have two ma6jor problems. (a) They don't match the numbers calculated repeatedly by multiple experienced scientists since Arrhenius 1896, and (b) they are directly contradicted by experimental evidence that supports the consensus views, see Harries et al 2001 satellite spectral data and following papers for that - doubling CO2 will directly increase temps by about 1.1C, with feedback increases in H2O and others bringing it to roughly 3C per doubling.
Empirical evidence flatly contradicts your claims - you might want to reconsider your assertions.
Kr @254, I appreciate your concern for the idea of reconsidering what my model may be lacking. It is well known that carbon dioxide is a substance that can trap heat by capturing IR photons. You are correct is pointing out how Svante Arrhenius correctly predicted this property of carbon dioxide. He did not predict the value, in terms of degrees/ppmv that it was capable of increasing the climate temperature. The attempt of scientists to determine a value or an approximation of this effect have relied heavily seem have relied mostly on a single approach. If this approach has the assumptions of Tom Curtis in 253, then there are some problems with it, as it seems to be in violation of the laws of quantum mechanics and thermodynamics. Tom Curtis in 253, mentions that there are 380,000 (millions) of collisions that an excited CO2 or H2O molecule will experience before it returns to it ground state energy level. This implies that there are millions of quantum transitions between excited state and ground state (which he incorrectly refers to as the base energy state); furthermore, it leads one to wonder it he also is implying that in each collision, there is a high energy transformation between the excited molecule and one of the other 990,596 ppmv that are not greenhouse gases, leading to closer than billion collisions. If this is indeed the implication, then it is a gross misrepresentation of the Kinetic Molecular theory, in which all collisions are elastic collisions. The first question that arises is, where the kinetic energy conservation? So the attempt to discredit my explanation falls short. You can't apply classical mechanics to a quantum mechanical problem. Remember the ultraviolet catastrophe? I guess in this case it would be the IR catastrophe. The foundations laid forth by great scientist such as Max Plank and Albert Einstein and Louis de Broglie, James Clerk Maxwell and William Thompson, (Lord Kelvin) can be used correct the apparent errors in in experimental procedure and calculation by the examples provided; examples that are being used to demonstrate the falseness of my model. I also read a comment either on my original post under the Trump Presidency, or in the earlier page 5 of this post that asked why I am posting to non-scientists. I also read a response (251) that somehow the observations contradict my assertions. I am going off the daily observations we experience from wallowing in the climate soup. My assertions are supported by these observations. One thing everyone forgot is the slightest variations in solar output influenced by sunspot activity. This will result in different observations in upwelling and down welling radiant differences from year to year, as have the most profound differences when observations were made between peaks and valleys of the 11 year sunspot cycles. The observations by taking the difference between the radiative upwelling, at the peak wavelengths of CO2, at surface level and TOA level is full of potential errors, because liquid water absorbs at this wavelength. Also
I thought this skeptical science site was a blog with scientists. I was told by a scientist at Oak Ridge Laboratories to find answers to my questions on the effects of CO2 on heating the climate, to come to this site. I have to admit if I told my students to research blog sites in order to find answers to their research questions, that I would probably get some flack by fellow teachers and even parents for suggesting such an idea. But the value I saw is that it would allow other scientific minds to critique my views. I have presented my paper to AJP and should probably wait until they are able to look it over. I do worry that if I made one little error in formatting or even grammar and spelling that they will throw it out without any response. I suppose I have learned a lesson as to get on a site and have many ad hominem insults added into the critique. I know that there need to be some calculations. I will forgo those until the paper is evaluated by scientists who review for AJP.
[JH] For future reference, if you want people to read what you post here, it would be extremely beneficial for you to break-up your lengthy tomes into a set of digestable paragraphs separated by spaces.
Yes the calculatios are in my paper.
Rudmop,
If you are going to calcualte the increase in temperature from an increase in CO2 it is useful to review those who have gone before you. Contrary to your claim at 255, Savante Arrhenius estimated that an increase in CO2 by 50% (from preindustrial 270 to 405 ppm) would result in an increase in temperature of 3.4C. Arrhenius 1896 page 267 (cited by 2015 other papers). This is a little higher than the current IPCC range of 2-4.5C for a doubling of CO2, although some current estimates are as high as Arhennius. Essentially, Arhennius is in agreement with current estimates while you are at odds with them. I note that the stratosphere was not yet discovered when Arhennius made his calculation although I do not know if it affects the calculation.
Please show why your model is so grossly different from Arhennius. Suggest where Arhennius was incorrect that everyone else has missed and you have found.
When you claim that Arhennius did not make this calculation you appear to be uninformed about what other scientists have done and how the calculation is made.
michael sweet @257, for what it is worth Arrhenius calculated the relative absorption by H2O and CO2 by comparing observations of IR radiation from the Moon by S P Langley under different humidity conditions, and latitude of observation, after correcting for different times of observation. By this times he determined the absorption across the whole IR spectrum when IR light passes through a "unit" of CO2 or of water vapour. A "unit" was defined as the amount of CO2 passed by a ray passing vertically through the atmosphere, for CO2; and a similar ray with humidity conditions at the surface of "10 grammes per cubic meter of the Earth's surface" (p 240), which are noted as being near average humidity. The effect of the different units for the different gases means that the measure automatically corrects for the different absolute abundences of CO2 and H2O vapour. With those units he finds (for one latitude, see page 245) that CO2 absorbs 6.6% of radiation per unit, while H2O vapour extinguishes 22.5% per unit, ie, that CO2 absorbs 20.6% of the total 20.6% of the total radiation absorbed under average humidity conditions.
The similarity of Arrhenius' estimate of the relative impact of CO2 to modern estimates under average humidity conditions helps explain his similar estimate of climate sensitivity. However, conceptually Arrhenius is still operating under a mistaken idea of the full mechanism of the greenhouse effect, and mistaken in a similar way to Rudmop. In particular, of necessity given the relative state of knowledge, he makes no allowance for the impact of altitude and temperature differences. Further, although his estimate is observational rather than theoretical, it is based on very low quality obervations relative to modern standards. As a result, his estimate overstates the relative impacts of CO2 and H2O absorption under similar conditions as estimated from LBL radiation models. That is because he considers only absorption, and not thermal emission.
That error is partly compensated for by his model of the greenhouse effect (p 254 forward), which of necessity uses the Stefan-Boltzman law, but not Planck's Law (which was not proposed till 1900). Further, it uses a single, near surface temperature for the atmosphere, which is in fact eliminated from his final formula for the greenhouse effect (equation 3). The result is a theory quite different to the modern theory of the Greenhouse effect as expounded by Manabe.
All this leads me to suspect the near approximation between Arrhenius' and modern estimates of climate sensitivity is to significant extent, a coincidence. I have not the mathematics to show that is the case.
Rudmop... "I also read a response (251) that somehow the observations contradict my assertions. I am going off the daily observations we experience from wallowing in the climate soup. My assertions are supported by these observations."
You have asserted this but have yet to demostrate it, and the opposite has been repeatedly demonstrated.
"I was told by a scientist at Oak Ridge Laboratories to find answers to my questions on the effects of CO2 on heating the climate, to come to this site."
That's nice to know!
michael sweet @ 257, You are correct there about Arrhenius’ estimates. I'm forgetful; I knew that and was in error in my statement. I find it funny I had to go to my book in order to find this. Can we agree that the difference in the pre-industrial revolution temperature anomaly and the temperature anomaly of today is +1.2 degrees Celsius, (understanding that the entire difference cannot be attributed to CO2, but in this situation we will ignore that important detail, since the current climate model ignores that important detail)? By the way, much of that 1.2 degrees in difference has been questioned in its validity, but that is for another topic which I address in my book, and will not address here. So the 3.4 deg. C from Svante's estimates was an over estimation by 183 %. (3.4/1.2*100)-100
Using the numbers you provided for the IPCC of between 2 and 4.5 degrees Celsius for the estimated temperature rise attributed to carbon dioxide, we get a reasonable error of 67 % high, and a huge error of 275 % high. If you take the error for the average of their two predictions, you get an error of 158% too high. Notice there is no under estimation, which there should be if we are going to realize that increasing carbon dioxide is not the sole contribution to the 1.2 degree Celsius (questionable) difference in temperature anomaly since the industrial revolution.
In my model, if you take [(.28 deg.C/404ppmv CO2)x135 ppmv increase in CO2], you get 0.094 value for the temperature contributed by carbon dioxide to the difference between temperature anomaly since the industrial revolution and today. This is 92 percent too low for the measured temperature anomaly.
[PS] It would be more convincing if you could explain how your calculation is compatible with the observations in say here. You must demonstrating a match to observations for any theory to have merit.
I am sorry I did not read the admin suggestion about breaking up my paragraphs with spaces. I need to write in a spell check mode, as I am constantly mispelling words. Since this has no spell check editor, I write in MS word and cut and paste. I was careless in realizing that it does not keep the formatting of paragraphs when you cut and paste. My style of writing is a heavy usage of the semicolon; I appologize for that, but it is an unbreakable habit I learned by my excellent English Teacher, Mrs. Fitch in 10th grade.
[PS] When most people write something, they want people to read it. Improving readability is absolutely worth moving on from you learnt in the 10th grade.
Rudmop... Here you continue to assert without offering any substance, math, or evidence.
Rudmop,
Tom's description of molecular collisions reads:
"The notion that a CO2 molecule "... cannot hold on to any trapped energy for an indefinite amount of time", while accurate, is irrelevant. Even at 85 km altitude (US standard atmosphere), an excited CO2 (or H2O) molecule will, on average, experience 380,000 (a million) collisions before it would typically have spontaneiously returned to a base energy state by emitting a photon. Within the troposphere the figure is closer to 5 billion collisions. Therefore absorbed radiation is rapidly transmitted to the rest of the atmosphere as heat, and stored by the whole atmospheric layer. The emissions from that layer, in turn, are almost exclusively from CO2 (or other greenhouse gases) that have entered an excited state due to collissions from with other molecules. That is why the emission fits the profile of thermal radiation (within the radiating wavelengths). And because the radiation is thermal, it is controlled by the temperature of the layer, not the rate of absorption of photons from lower layers in the atmosphere."
This means that if a CO2 molecule absorbs an IR photon of energy it takes some time before it emits that photon again. Let us say it is a microsecond (you can Google the actual time). In that time it will undergo a certain number of collisions. Let us say it is 5 billion collisions (as per Tom Curtis' data above). Since the IR energy can be dissipated by collisions with other molecules it is approximately 5 billion times more likely that the CO2 molecule spreads the IR energy around to other molecules through its collisions than that it re-emits another photon of energy. No quantum magic is involved. In the end the CO2 molecules in that section of the atmosphere emit IR radiation proportional to their temperature as required by Quantum Mechanics. The amount of energy they absorb from lower in the atmosphere is irrelevant (except that it increases the temperature). This is the basic cause of the greenhouse effect.
Your discussion of quantum states, Kinetic Molecular theory and Albert Einstein appears to be an attempt to divert attention from the fact that you do not understand how energy is transmitted through the upper atmosphere. This mechanism has been discussed several times here at SkS. If you ask nicely there are scientists who post here who can explain it to you, including Tom Curtis. If you do not understand the basics you need to study more before you claim that you have discovered that everyone else is incorrect. Before you make public presentations on the greenhouse effect you must understand how energy is transmitted through the atmosphere.
The quality of your comments is going down. You provide little data to support your claims.
IPCC estimates 2.0-4.5C per doubling. Since CO2 has only increased 50%, there is at least 0.6C warming in the pipeline and 1.2C (from you, I prefer 1.5C) has been measured, I calculate the current warming as 1.8C. That means that the low end of the IPCC significantly underestimates current warming and the high end might be about right. You are mistakenly comparing current warming to IPCC projected warming with 540 ppm CO2. I note that these temperature changes do not include most of the long term effects of melting ice and snow (which add to the warming).
If your book challanges the warming observed by everyone, you will need data (which you have not provided) to suppport your wild claims.
For the record, I have a Masters of Science in Organic Chemistry and teach Advanced Placement Chemistry in High School and basic Chemistry at a local Junior College.
Keep in mind why you were sent here. A scientist realized that you have a lot to learn about atmspheric chemistry and commenters here have a strong reputation. Tom Curtis is the most active poster here at SkS. His posts are always detailed and supported by links to the appropriate literature. I recommend that you listen very carefully to anything he posts. If what Tom says does not make sense you should presume you do not understand the science. Do not think that Tom is incorrect without strong, peer reviewed data (which you have not produced).
Tom,
Thank you for the information on Arhennius. I now understand a little more about how he did his calculations.
Rob Honeycutt, in my percentage figures, that are based upon a questionable temperature anomaly, not my own value, but IPCC's value, I have supplied the elementary math.
If you are referring to the value of .28 degrees that I arrived at, here is what I did. I calculated the concentration ratio for CO2:H2O. I used an average value of 404 ppmv for CO2 and a value of 8000ppmv for H2O. Both of these figures are very reasonable figures. I am not going to do the gas law calculations to show how I arrived at such an agreeable figure for the average water vapor concentration. The math you are requesting is 404 ppmv CO2/8000 ppmv H2O Vapor and the answer is 0.051.
I used Grahams Law of Effusion to calculate the ratio for the diffusion rates of CO2:H2O vapor. You can do this and you will get a ratio of 0.64 for the rate of diffusion of both gases.
If you are looking for big calculus equations, perhaps you can apply an integral to the absorption spectra of carbon dioxide and water vapor to calculate the absorption energy of an excited molecule of carbon dioxide and an excited molecule of water vapor in superposition. I took the integral for the wavelenght values 14.8 um to 15.2 um for carbon dioxides IR spectral absorption. I also took the integral of its spectral absorption over the wavelengths from 4.3 um to 4.0 um. Since water vapor will emit wavelenght in the range IR wavelength 2.4 to 3.0 um when it undergoes deposition and condensation respectively, I integrated the energy absorption for carbon dioxide at these wavelenghts. For water vapor absorption energy, I integrated the energy absorption values for the wavelengths from 15um ot 27 um and 2.5 um to 3.4 um, noting that even though the blackbody radiation almost entirely excludes the wavelenghts 2.5um to 3.4 um, the fact that the exothermic phase changes of water during depostion and condensation will emit these wavelenghts. Finally I integrated the values for the IR absorption energy from wavelenghts 4.9 um to 8.0 um for water vapor. Please not that I do multiply by a factor of .125 to the integration result from wavelengths in the 4um to the 7 um range because the blackbody emission of these wavelengths is so low. The absorption energy ratio of CO2 to H2O is 0.27
Multiplying all these coefficients together to determine the absolute coefficeint for the ratio of how many time CO2 can absorp and transfer heat as compared to water you get 0.0087. Multiply this by the of the temperature above blackbody temperature that these gases maintain (32.8 deg. C), and you arrive at the answer of .29 degrees for carbon dioxide and 32.51 for water vapor.
I know this approach does not use differences in ground level radiance and TOA radiance for peak absorption wavelenghts of CO2. It is my asserction that since liquid water can absorb at the 15 um wavelengths then some of these wavelengths will be blocked by water droplets on the surface of condensation nuclei in clouds. There will be no way to determine what percentage of these waves are blocked by CO2 and water in the clouds.
Rudmop @255:
No, I indicated that in the mean time to return to the ground state if there is no interference, around 380,000 collisions will have taken place, each of which has a significant probability of returning the molecule to the ground state with the excess energy either causing the other molecule (if of the same type) to enter an excited state, or more probably, with the excess energy being converted to kinetic energy. At the same time, each of those collisions also has a low probability of causing a molecule in the ground state to enter an excited state. The result is that radiation from the gas will be thermal radiation, ie, radiation described by Planck's Law.
You to wonder that, but there is not basis in what I said for that speculation. There is a high probability in each collision that the energy of the excited state if retained to that point, will be transferred to kinetic energy. But if transferred, the molecule is then in the ground state, and cannot transfer the energy a second time on subsequent collissions (as you are suggesting).
The Kinetic Molecular Theory (or the Kinetic Theory of Gases), is an ideal theory known to be false in its assumptions. In particular, of the assumptions (below), assumptions (1), (2), (4), and (5) are known to be false of real gases. Unless you are prepared that all molecules are spherical in shape (assumption 1), that they are not subject to gravitational acceleration (assumption 2), that they are not subject to van der Waal's forces, or the strong and weak forces (assumption 4), you have no right to insist that assumption (5) obtains in actuallity, rather than in approximation.
Further, there is no theory of the conservation of kinetic energy. There is a theory of the conservation of energy, but nothing I have said suggests it is violated (unlike your speculation).
What is worse for your argument is that you have already agreed that IR radiation captured by CO2 raises surface air temperatures, however minimally. It follows that they increase the mean kinetic energy of the near surface atmosphere, ie, violate the non-existent law of conservation of kinetic energy. It also means that they convert radiant energy to osciliatary (or rotational) energy, which is then converted to kinetic energy by collisions. If there was any validity in your argument, it would refute your own theory.
Your attempts to justify your theory in the face of criticism are becoming increasingly desperate and ridiculous.
Rudmop... I trust that you've gone through a large number of calculations to get to a result. That's not the question at hand. The question would be, do your results explain observations?
If it doesn't, sorry. No Nobel Prize.
Tom, I appologize for misreading your statement. I see what you are saying now; thankyou for the clarification. If we both agree on how heat is transferred by the greenhouse gas molecules in the atmoshpere, more than 100 meters above the surface, by first absorbing IR photons, and then transferring the energy via elastic collisions, then it should be evident that the number of collisions that can occur between water vapor and all other molecules and carbon dioxide and all there molecules will depend on concentraion and velocity. This is an important part of my model.
It will also depend on the amount of IR energy absorbed by water vapor and Carbon Dioxide molecules. These three ratios (concentration, diffusion rate, and IR absorption energy) are the meat and potatoes of my model.
Rob Honeycutt, @268,
If you walk outside right before sunrise tomorrow, and you experience a temperature of -18 Celsius, then you will be able to tell me if my results explain my observations and yours. On the other hand if you walk outside right before sunrise and the temperature is 32.8 degrees warmer than the blackbody average for your latitude, then my results will explain your observations. 0.87 percent of this temperature is due to the contribution that CO2 provides in trapping in surface heat. The remaining 99.13 is due to carbon dioxide. If you would like to factor in the ground level ozone, N2O5, CH4 then the values will change a bit.
Sorry, the other 99.13% is due to water vapor. I'm out now.
Sorry, bubba. You can't just claim reality is your observational evidence.
Try this. Think of a specific observation that would demonstrate that CO2 has a tiny impact on global temperature, as you suggest.
The rest of the scientific community has done this in spades over the past century. Exit from snowball earth events. Early faint sun paradox. Silicate rock weathering. Temperature excursions with the Siberian/Deccan traps. Etc.
Or, alternatively, apply your theory to Venus. Tell us what your equations output for the surface temperature.
And honestly, if you didn't do this in the paper you've already submitted... I don't think you'll get past the waste paper basket at the front desk.