How substances in trace amounts can cause large effects
What the science says...
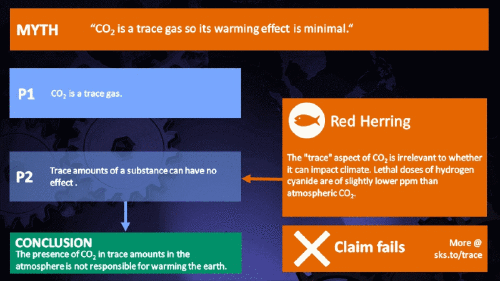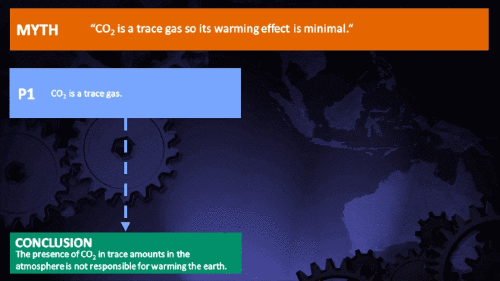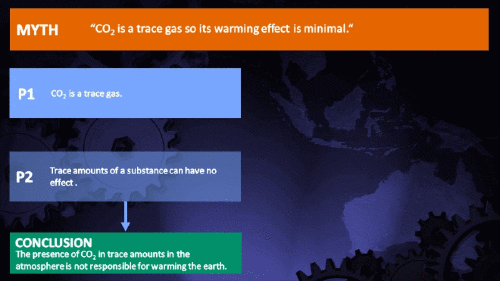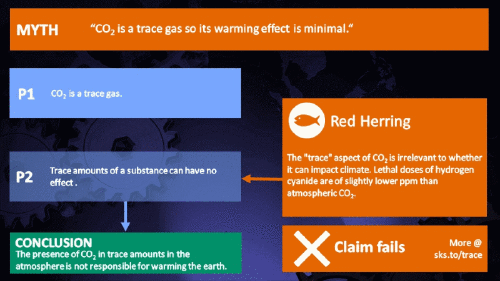
Small amounts of very active substances can cause large effects.
Climate Myth...
CO2 is just a trace gas
"We have been grossly misled to think there is tens of thousands of times as much CO2 as there is! Why has such important information been withheld from the public? If the public were aware that man-made CO2 is so incredibly small there would be very little belief in a climate disaster ..." (Gregg Thompson)
At a glance
When the first edition of this rebuttal was posted in August 2011, atmospheric CO2 was at around 390 parts per million (ppm). Now (August 2023) the number is 421 ppm - and rising.
To a non-chemist, 421 ppm might sound like a vanishingly small amount, simply because you're comparing a small number (421) with a very big one (1,000,000). It therefore comes as no surprise to find that this apparent contrast was seized upon by practitioners of misinformation. Claiming that things occuring in apparently tiny amounts must be harmless is such an easy talking-point to bandy about, since much of the intended audience is unlikely to deal with such numbers on an everyday basis. But it's also one great big red herring. Why?
Hundreds of parts per million may not sound like a lot, but in fact many substances have important properties when present at such levels. Here are a few examples:
- He wasn't driving drunk, he just had a trace of blood alcohol; 800 ppm (0.08%) is the limit in all 50 US states and limits are even lower in most other countries.
- Don't worry about your iron deficiency, iron is only 4.4 ppm of your body's atoms.
- That ibuprofen pill can't do you any good; it's only 3 ppm of your body weight (200 mg in 60 kg person).
- The Earth is only 3 ppm of the mass of the solar system.
- Your children can drink that water, it only contains a trace of arsenic (0.01 ppm is the WHO and US EPA limit).
- Ozone is only a trace gas: 0.1 ppm is the exposure limit established by the US National Institute for Occupational Safety and Health. The World Health Organization (WHO) recommends an ozone limit of 0.051 ppm.
- A few parts per million of ink can turn a bucket of water blue. The color is caused by the absorption of the yellow/red colors from sunlight, leaving the blue. Twice as much ink causes a much stronger color, even though the total amount is still only a trace relative to the water.
- Only 500 ppm of hydrogen sulphide (bad egg gas) in air is a hazardous level, as any health and safety fact-sheet will tell you. It will make you seriously unwell at that kind of concentration. In fact, at above 100 ppm, you can no longer smell the gas because its toxicity has switched off your sense of smell.
Just a trace-gas? Yeah, right.
It's not the concentration of a substance that necessarily matters. Instead, it's what any substance can do at a certain concentration and that property will of course vary from one substance to another. These are all useful things to recall when someone dismissively tells you that carbon dioxide is “only a trace gas”. It doesn't matter.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
Although percentage or parts per million are convenient ways to talk about the relative amounts of gases in the atmosphere, they only tell us how much there is compared to everything else up there. Concentration doesn’t give an absolute amount.
For example, most people will have trouble breathing on top of Mount Everest. The atmosphere still contains 21% oxygen, just like at sea level. But the air is rarefied, meaning the relative proportions of all of its constituent gases are the same but the density of their molecules has reduced by around two thirds. With every breath you take you will only get around a third of the oxygen molecules that you would at sea-level. It's the number of oxygen molecules that matter because through their chemical properties and interactions with our bodies, they support respiration - and therefore life.
We are now emitting some 44 thousand million tonnes of carbon dioxide every year (source: IPCC AR6, WG 3 Technical Summary 2022). Fourty four thousand million tonnes is a hard number to visualise. One way to do so is to consider that it's more than seventy times heavier than the combined weight of the current global human population. Maybe that's even more mind-boggling! But nitrogen makes up 78% of the atmosphere - it's vastly more abundant than CO2. Because of that, the concentration of CO2 - despite such vast emissions figures - just nudges up by a few ppm a year.
In 1959 the measured CO2 concentration was 316 ppm whereas at the time of writing (2023) it's 424 ppm. That represents an exponential increase of 108 ppm over 64 years - exponential because our annual emissions have now increased vastly, compared to the 1950s and 60s. That's despite the knowledge of the harm being done by them.
Pre-industrial levels of CO2 were around 280 ppm, so the overall increase to date has amounted to some 144 ppm - a 50% increase. That CO2 increase has in turn been sufficient to raise global temperatures by more than a degree celsius relative to pre-industrial times, so we have a bench-mark with which to look at future emissions. Furthermore, because CO2 is a non-condensing greenhouse gas, once it's up there in the atmosphere it stays around for a long time, so much of that 144 ppm increase still has work to do - meaning that the warming already caused by it will continue for many years.
Often we talk about a doubling of CO2 from pre-industrial levels (280 increasing to 560 ppm) and its effect upon temperature: Arrhenius did the first calculation on this over 100 years ago. He could not foretell a time when such a CO2 overloading might occur due to human activity, since the emissions of the very early 1900s were very low compared to now. We now have that doubling firmly in our sights. In the decade of 2013-2023, CO2 levels have risen by nearly 30 ppm. If our emissions flatlined at current rates, we would reach that doubling-point sometime in the 2060s - not that far off at all - and that's assuming there are no nasty surprises waiting for us in terms of feedbacks.
So we know the amount of CO2 in the atmosphere has increased in this manner because we have constantly measured its concentration since the late 1950s. We know from current and historical data that the climate has already significantly warmed,. The link between increasing greenhouse gases and increasing temperature is even clearer now than it was in the 19th Century. CO2 in our atmosphere is trapping energy that would otherwise escape to space. That's an intrinsic property of this particular substance.
If a trace gas can absorb and re-radiate IR at even low concentrations, what do you think will happen if we double that concentration? Twice as many CO2 molecules, all busy doing precisely what they do and for long into the future. CO2 may be a trace gas but because of that intrinsic property it has, that doesn't matter.
Cartoon summary

This Cranky Uncle cartoon depicts the "Red Herring" fallacy for which the climate myth "CO2 is just a trace gas" is a prime example. It is used to deliberately divert attention to an irrelevant point to distract from a more important point. Please see the accompanying blog post for more information about the cartoon collection.
Last updated on 13 August 2023 by John Mason. View Archives































 Arguments
Arguments






























Is the trace gas thing still alive?
Anyway, for a trace gas, it's amazingly important for life on the planet. Such tiny amounts supply us with food, trees etc.
Plus, if you just look at the greenhouse gases in the atmosphere (like IR radiation), CO2 is the second most abundant, currently at about 9%.
"Small amounts of very active substances can cause large effects"
Yes. But only if that substance is "very active".
IS CO2 indeed "very active"?
it only reflects IR "black body" radiation. therefore is it not very active in terms of effecting heat, which spans a much longer band of wavelength than just bloack body IR.
Even within the IR black-body band, CO2 only effects three tiny absorbtion bands that account for 8% of the "black body" wavelenths.
Correct?
If this is correct, CO2 is 100% not "a very active substance".
Rather a better description is that it is a "very weak" reflector of heat.
Correct?
Water is a far more powerful green house gas both because of the level of heat it is able to reflect, but also the massive concentration of H2O in the atmosphere.
Correct?
back to CO2 - when you combine the fact that it seems to be a very weak reflector of the overall heat spectrum, combined with the fact it is present by concentration 0.03% of air, surwely the climate change movement might have made a massive mistake in placing CO2 as the central cause of global warming?
....when you heat water, it releases gasses including CO2, hence could CO2 in past warmings be an effect rather than a cuase?
[Rob P] Allcaps removed. Further transgressions will result in comment deletion.
Undecided Molecular Biologist The basic mathematics of the enhanced greenhouse effect was worked out by Gilbert Plass back in the 50s, and the calculations were based on the "small" amount of the IR spectrum that CO2 actually absorbs that you mention. The reason this can have a great effect is that the sun provides a very large amount of energy into the climate system, so (loosely speaking) you only need a small proportional change in the amount that escapes can have a big effect on surface temperatures.
I suggest you get a copy of Pierrehumbert's book "Principles of Planetary Climate", and follow the maths, and you will find out how the greenhouse effect actually works and you will understand how important these small absorbtion bands are.
UMB, what sort of "If by Whiskey" argument is that? The relative strengths of the various greenhouse gases have been directly measured (example) from surface. You know that, of course, and so I'm wondering why you're engaged in semantics when you could be going through the math. If you want to talk about tiny changes, why not point out that if we use the full Kelvin scale up to the max GMST for the last 550 million years, a change of -3% results in a massive ice age. A change of less than 0.2% resulted in the LIA.
You might also check out this series of articles (the author welcomes feedback from those able to do the math).
"reflector"?
You both refer to mathematical "proof" that CO2 is indeed a "very active" substance via models. This probably risks needing to move the subject to another thread, but to continue the point -
Pierrehumberts book states very aggressively in his opening remarks, just one (of potentially thousands) of flaws in climate model calculations. Namely that water, or indeed clouds, pose a very severe challenge to the understanding of climate. One calculation error on either side of the effect clouds have upon radiative forcing, will destroy a model.
When the number of interacting variables in a model reaches numbers that clearly climate science does, they have to be wrong, they will be wrong. Pure common sense says this. Indeed, you can back-model climate to check if you are right, but that is including the known variables. Bankers back-modelled AAA rated financial products 5 years ago. There was overwhelming consensus that they were right in their own (greedy-world) of peer reviewing each other’s work. Trillions were invested "risk-free".
To model the risk profile of a AAA rated asset backed collection of securities is a piece of cake compared to trying to model climate science. And what happened?
They were wrong. They missed a simple variable and the model broke. Trillions lost and global recession we are still feeling the effects of. Big mistake by an overwhelming consensus at the time and by (simple by comparison) models being wrong.
I worry that the climate movement has made a grave mistake in backing CO2 as the driver of climate change....
If it is proven to be a mistake, public will lose confidence and trust in the environmental movement and I fear even more important issues such as habitat loss, population growth, antibiotic use and sustainable practices will get effected.
This is my big fear
[JH] You are now skating on the thin ice of sloganeering and excessive repititon -- both of which are prohibted by the SkS Comment Policy. Please cease and desist, or face the consequences.
[Rob P] Allcaps removed. See comments policy.
Undecided Molecular Biologist:
There's nothing for it but to say that you appear to be operating with an extraordinary misconception of the underpinnings of climate science. Just imagine some random person coming along and spouting off completely off-base stuff about molecular biology. That is what your comment #8 looks like with respect to climatology.
Climate models are emphatically not the underpinning of climate science. If anything, they're latecomers to the game. Climate science begins with the paleoclimatic studies of ice ages and the experiments of Tyndall in the 19th century, not with the hi-falutin' models discussed in IPCC reports.
Our present understanding of climate and of greenhouse gases follows, of necessity, from the physical properties of greenhouse gas molecules themselves and their IR-radiative behaviour. As far as I am aware, these properties were more or less completely determined in the 1950s and 60s.
The understanding that CO2 is a critical forcing while H2O is a feedback (that is, H2O does not force climate changes, it can only amplify them) also follows of necessity from the same physical properties.
What is more, we have access to empirical data from paleoclimate research and recent records-keeping, which we can use to validate modelling. As far as I am aware the bulk of empirical data strongly supports the mainstream understanding of climate.
I have nothing to say about your attempt to draw an analogy between climate modelling and a particular set of financial modelling (if indeed you have characterized the latter accurately) or your final remarks, which IMO amount to issue-trolling, however well meant they may be.
As a follow-up to my comment #9, I should like to add the following summary:
The claims of denialists notwithstanding, the fact of the matter is, like any well-validated science, climatology is backed up by the three-fold combination of:
(1) Theory - the known physics of radiative transfer, bulk heat transfer in the atmosphere & oceans, the radiative properties of greenhouse gas molecules, etc. etc. etc.;
(2) Experiment - e.g. Tyndall's work in the 19th century, modern climate modelling, etc.; and
(3) Observations - e.g. satellite era research showing the top-of-atmosphere energy imbalance, ARGO floats finding immense increases in ocean heat content, global cryosphere melt, and so on (and on and on...)
I can assure you, Undecided, and any other readers, that the findings of climatology cannot be so easily tossed aside by casual references to bad financial modelling in the last decade: any attempt to overturn it has to come to grips with the theory, experiment, and observations.
undecided The comment in Pierrehumbert's book refers to clouds (which do not contribute to the greenhouse effect), not water vapor (which does), and so does not in any way support your earlier comments regarding H2O versus CO2.
Pierrehumbert, like many climatologists, is perfectly happy to talk about the limitations of the models, however he are still willing to use them. The fact that this is the case should give you pause for thought, that just perhaps you are blowing the limitations out of all proportion, and that perhaps you need to actually read the books and papers that explain how the models work, rather than just read the opening remarks until you find a comment that you can use to support your position.
Undecided Molecular Biologist: To get the discussion onto a more productive footing, do you agree that the theory of the enhanced greenhouse effect (see e.g. here for a brief explanation of the basic mechanism) that predicts warming as the result of increases in atmospheric CO2 is based on the recognised absorption characteristics of CO2 that you have mentioned?
UMB - Follow the logic train;
#11:
http://skepticalscience.com/argument.php?a=185#98478
"The comment in Pierrehumbert's book refers to clouds (which do not contribute to the greenhouse effect)..."
I was under the impression that clouds did indeed contribute to the greenhouse effect, though the total forcing from them is negative when you take into account the increase in albedo. The cloud feedback due to temp changes is thought to be most likely positive, though that is not certain. I do however understand that UMB misread
Pierrehumbert, who as you say was talking about the uncertainty in the cloud feedback. That's not true with the water vapor feedback, which we know is strongly positive.
Robert: how do you define "greenhouse effect"?
Clouds do asborb IR. But the greenhouse effect is traditionally thought of as the atmospheric effect where the atmosphere is transparent to visible sunlight, and relatively opaque to IR. Radiation from the sun reaches the surface easily, but is impeded on the way back out. As you note, clouds are not particualrly transparent to visible light. Thus, by my definition, they are not part of the greenhouse effect.
Undecided: "You both refer to mathematical "proof" that CO2 is indeed a "very active" substance via models."
You have hand-waved away the effect of CO2 by using vague, ill-defined terms such as "not very active", "tiny absorption bands", and "very weak". Your "proof" is nothing more than an assertion.
People are trying to point out to you that when you actually put numbers on "not very active", "tiny absorption bands", and "very weak", and do the math, the result says that the amount of CO2 in the atmosphere is actually important. It really does affect the radiation balance, and it really does increase global surface temperatures.
You may think that handwaving trumps a mathematical calculation. Science generally takes the opposite view.
Undecided Molecular Biologist @5 & 8, here is a spectrum of infrared radiation to space at the Top of the Atmosphere as calculated by the Modtran Model:
The important points are:
1) The Earth's TOA black body radiation without a greenhouse effect would follow the shape of the coloured lines (black body radiation curves), with the specific shape depending on surface temperature;
2) The absorption of IR radiation from the below, and reemission at a higher cooler altitude results in a reduction in the TOA outgoing radiation, by the amount shown by the red shading;
3) The largest single factor in that reduction is H2O with absorption and reemission at wave numbers less than 550 and greater than 1300 (the initial dip around 1250 is due to methane);
4) The second largest single factor in that reduction is that due to CO2 at a wave number of about 650;
5) The reduction to CO2 is almost as large as that due to H2O in a clear sky;
6) Although there is some overlap of H2O absorption and CO2 absorption, because CO2 is higher in the sky (as can be seen by its lower temperature of emission), it would have the same effect even in the absence of the H2O, so that the H2O has no effect in areas of overlap; and
7) The large CO2 absorption band is located near the peak of terrestial emissions allowing it to have a much larger impact than other absorbers.
Modtran is only a model, so you may be disinterested in what it shows. Such models have been compared with observations, however, and shown to be remarkably accurate. An early such comparison was published in 1969:
These and similar observations show that your parade of "corrects" are based on prejudicial thinking rather than on actually looking at the observational data on the issue. Absent such prejudicial reasoning, it can be discovered that CO2 is responsible for approximately 20% of the all sky greenhouse effect.
Bob Loblaw @16, your definition is non-standard.
The best definition of the atmospheric greenhouse effect is the difference in upward longwave radiation at the TOA to that at the surface due to absorption and emission of longwave radiation be components of the atmosphere.
Based on that definition, clouds contribute approximately 25% of the total current greenhouse effect, coming in behind water vapour (50%) but ahead of CO2 (20%). (See link in my post responding to Undecided Molecular Biologist above.)
Tom:
My definition is the essential of the old reasoning that led to the (poorly-chosen) name "greenhouse effect". The idea was that the glass of a greenhouse let in visible light and block IR going back out. There is the old "re-radiation" line of thought that builds into that as well. So, like a greenhouse, the atmosphere lets in visible light and blocks IR.
Now, it turn out that greenhouses are not warm because of the blocking of IR - plastic ones transparent to IR work just as well - but rather due to the greenhouse confining heat close to the surface by reducing turbulent mixing of the air. So, the radiative effect of the atmosphere isn't at all like a greenhouse. [And I know you know that.]
But then, it turns out, thinking solely about the radiative effects of the atmosphere also doesn't really explain it all, either. If radiation were the only way of moving energy around, the atmospheric temperature profile would be a lot different from what it is - with a much warmer surface. But the atmosphere is mixed, and much energy is carried from the surface to the upper atmosphere by thermal mixing and by evaporation (at the surface) and condensation (at height). [And I know you know that, too.]
So, neither the greenhouse, nor the atmosphere, are explained by soley the IR radiation characteristics.
I disagree that a definition of "greenhouse effect" that only looks at IR radiation is "best". If the atmosphere was opaque to visible light, then the top of the atmosphere would be hottest, and IR wouldn't matter much at all. After all, look at the stratosphere: just the extra absorption of energy in the UV range is enough to reverse the temperature profile. The fact that much of the energy from the sun reaches the earth's surface is an essential part of the process.
Until I try to access a copy through work, I'll have to settle for the abstract of the paper you reference. Although I understand the need to correct distortions of the radiative effects of various constituents that are presented, the paper does just appear to focus on the IR radiation portion of the issue. That's enough to show the bogosity of "it's all water vapour" crowd, but it's still an incomplete picture.
Tom:
I have managed to download the full paper you refered to, and I gave it a quick read this evening.
Although I agree with your summary of the contents of the paper, and I agree that it is a very useful way of quantifying the relative importance of various atmospheric constituents, I still contend that "the Greenhouse Effect" writ large must include consideration of the atmospheric transparency wrt solar radiation.
Two interesting aspects of the paper:
1) the dual approach of adding consituents one at a time to the model, verus subtracting them (with others prreset). Various constituents have overlapping absorption bands, which are accounted for in the radiation code. Adding consituents one at a time and watching the changes tells the maximum effect (as any "overlap" won't be an overlap). Removing them one at a time leaves the overlap active in the remaining constituents, and shows a minimum effect. THis puts bounds on the range of values.
2) the use of a 3-d climate model gives a more realistic account for the spatial effects, compared to other estimates that used 1-d models. The exact effect of any constituent depends on local effects of temperature, cloud cover, etc. As a 1-d model can only deal with a single "average" condition, it is more limiting than the 3-d model approach.
Am I suffering from DPD (decimal point shift) or are we all missing a trick with this 'CO2 is just a trace gas' myth-busting? Are we missing an argument with a wow-factor-par-excellence?
I have just been reading a screed on vaccuum, how in the most empty bits of distant space the atoms (or more correctly the bits of atoms that exist in the WHIM) are about half a metre apart. The screed contrasted this separation with the comment that out atmosphere has atoms spaced at about one million per millimetre which is getting down to the sort of distance similar to the diameter of atoms.
So here we go:-
There is 400ppm CO2 in the atmopshere and it requires 2.13Gt(C) to add a further 1ppm. So there is 852 Gt(C) = 8.52+e17 g(C) contained within atmposheric CO2.
If this is divided by weight (C=12) and multiplied by Avogardo's number, we obtain a number for the molecules of CO2 within the atmosphere. 8.52e+17 x 6.022e+23 /12 = 4.28e+40 molecules CO2.
An atom has a rough diameter of 0.3nm. Thus it has an area roughly equal to 7e-20m^2 and a sheet of carbon made from the carbon content of atmospheric CO2 would have an area of something like 6e+19m^2.
As the area of the Earth is 510 sq km = 5.1e+14m^2, this means any point object attempting to exit the planet Earth from ground level (straight up in a straight line would be the shortest route) will have to pass through the middle of (6e+19/5e+14=) 120,000 molecules of CO2, even if they were aligned edge on and only showing an area equal to one of its atoms.
So it appears correct to say that, while there is only a small % of CO2 in the atmosphere, the (lower) atmosphere is quite well packed with molecules and molecules are very small. So there is a very large number of molecules in the atmosohere and, even if they are only a small % of the atmosphere, there is still a very large number of CO2 molecules within the volume of the atmosphere.
For a photon to travel the 7 miles or so to reach the stratosphere, it will pass through a very large number of atoms and a lot of them will be the atoms of CO2 molecules. To make such a journey a photon would have to negotiate its way through the middle of something like 120,000 CO2 molecules. If the wavelength of that photon is the sort that has a problem passing through CO2, any one of those 120,000 CO2 molecules could be the one that grabs it and brings its outward journey to a halt.
Coincidently, clouds are about 0.04% water. I've noticed quite a difference between sunny and cloudy days.
The discussions on this page are disappointing - childish word games. The only question that matters is: "does throttling a trace gas CO2 result in human control of the climate and weather." - a global thermostat. The answer is “no”, so the entire global warming fraud and everything about it is irrelevant – including solar /wind power and electric cars and all the green marketing. None of it is relevant. Unfortunately this post was too late to save the 100,000 US coal minors who lost thier jobs.
[TD] Provide a peer reviewed reference for your unsourced assertion that 100,000 coal miners were illegally underage.
[PS] This post is nothing but sloganeering. This is a science-based site. You must provide supporting evidence preferably from peer reviewed literature to back your comment. Opinions based on your preference or political leaning have no place here. You may find rants like this more welcome on sites like WUWT.
So are the non-greenhouse gases completely transparent to infrared?
Would an infrared photon go through an infinite amount of an oxygen, nitrogen, and argon atmosphere?
If so, I guess adding GHGs would definitely have an effect, regardless of the size of the atmosphere in question. If not, then the non-GHGs present would have an effect, and the GHGs could prove redundant.
Rovinpiper @24,
Answering your two questions, (1) yes non-GHG gases are transparent to the Earth's infrared and (2) O2, N2 & Ar which comprise 99.95% of the dry/clean atmosphere are non-GHG gases, so yes, presumably to infinity and beyond! But note that O3 (ozone) absorbs IR of 9.6micron wavelength and so oxygen in the form of O3 is a GHG.