State Department cuts through the acid political environment on oceans and climate
Posted on 29 July 2014 by Sarah
Secretary Kerry hosted a remarkable conference in June called simply Our Ocean. It enlisted international policy makers, scientists, and the private sector to take action to ensure a healthy ocean for the future. The conference laid out the science, impacts, and solutions for three critical ocean problems: sustainable fisheries, ocean pollution, and ocean acidification. A key goal was to alert those at the highest levels of government to the perils facing the ocean and to inspire them to act decisively to change course.
The normally staid State Department conference room was bathed in oceanic blue light and during breaks gentle whale songs played while whales and schools of fish swam across three walls of high resolution screens. An exhibit area featured NOAA's Science on a Sphere and NASA's Hyperwall both showing detailed movies of our dynamic planet.

NOAA Science on a Sphere. Photo from the Space Foundation.
The session on ocean acidification demonstrated that the adverse effects of carbon dioxide emissions are not limited to heating the planet. The acidity of the ocean has already increased by 30% because of added carbon dioxide. Simple chemistry says that trend will continue as long as extra carbon is added to the atmosphere. Bill Dewey of Taylor Shellfish Farms explained that oyster farmers on the U.S. West Coast are already adjusting their water management to minimize the damage caused by acidic waters. The COE of the Palau International Coral Reef Center, Yimnang Golbuu, showed that certain types of coral seem to be resistant to acidic waters, which may allow us to buy time for some reefs while we work to limit the extent of acidification. We also saw alarming photos of tiny sea butterfly (pteropod) shells that are already developing poorly because of acidity.
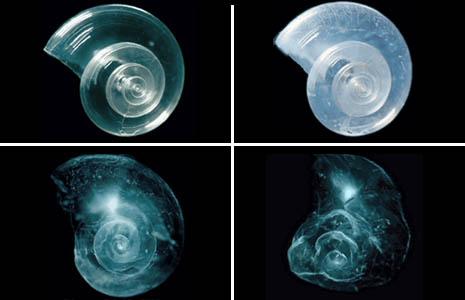
In a lab experiment, a sea butterfly (pteropod) shell placed in seawater with increased acidity slowly dissolves over 45 days. Photo: Courtesy of David Littschwager/National Geographic Society
The panelists made clear that acidification is a one-way street; once carbon dioxide makes its way into the sea, we cannot get it out.
Unlike marine pollution and overfishing, which require multifaceted solutions, ocean acidification has only one primary cause: excess carbon dioxide in the atmosphere. It therefore has an obvious solution: limit carbon emissions.
As Secretary Kerry said, the challenges of climate change and ocean acidification "actually have pretty obvious solutions that are staring us in the face. It’s not as if we’re sitting around scratching our heads saying, 'How do we solve the problem?' It’s really a question of, “How do we find the political will?'"
Political will comes from demands of citizens. But we need to apply pressure now because any political backbones that were strengthened by this conference could begin to dissolve in an acid political environment.































 Arguments
Arguments






























I have a problem with the theory that molluscs and corals are suddenly at risk from ocean acidification due to CO2 at 400ppm (though I don't doubt that they are threatened by many other man-made risks).
These two life-forms evolved over the last half billion and quarter billion years respectively.
Over almost all of that time period, atmospheric CO2 levels were much, much higher than they are today.
"Simple chemistry" would imply lower pH levels in the geologic past, far below what we're likely to cause with CO2 emissions. And yet, molluscs and corals survived and are still with us.
I can't explain it.
(Rob P) Actually the basics are rather straightforward. As far as many marine calcifiers are concerned, it is calcium carbonate saturation state that poses the strongest control on shell building - not the excess hydronium ions (low pH). Carbonate ions are one of the building blocks of calcium carbonate shells/skeletons, and one of the chemical reactions that takes place when CO2 dissolves into seawater is the lowering the carbonate ion concentration (technically activity).
Change in atmospheric carbon dioxide on geological time scales allows enhanced chemical weathering of rock to supply carbonate and bicarbonate ions back to the ocean. Furthermore, the total dissolved inorganic carbon (DIC) in the ocean undergoes a large increase due to the increased weathering that accompanies the ramped-up hydrological cycle (lots more rain dissolving lots more minerals into the ocean).
The net effect is an ocean very hospitable to calcification despite the low pH. The Cretaceous Period (the 'K' symbol in your graphic) is a classic example. Cretaceous is derived from the Latin word for chalk, as in the huge chalk deposits that formed during that time. These chalks deposits, such as the White Cliffs of Dover, are of coccolith shells - tiny marine plankton that thrived in the Cretaceous. Ginormous shellfish, Rudists, were the dominant reef builders of that time too.
During times of geologically-rapid increases in CO2, such as now, the ocean carbonate system can't keep up and the oceans become corrosive. Carbon dioxide dissolves rapidly in the ocean, but there is an insufficient increase in the rate of chemical weathering because it takes millennia for the enhanced rainfall to flush sufficient carbonate & bicarbonate ions back into the oceans. The sum effect is that ocean pH and calcium carbonate saturation decline in tandem.
This is why ocean acidification (corrosive seawater) only develops with geologically-rapid increases in atmospheric CO2, but doesn't otherwise. I've simplified this a bit, e.g. leaving out dissolution of carbonates on the ocean floor during periods of lowered atmospheric CO2, but that's the general picture. SkS will have rebuttals to this common misconception in the near-future.
Sure and freshwater molluscs can survive with pH as low as 5. However, the problem is the rate of change. OA requires higher energy levels from organisms to do calcification and that requires adaptions. When OA is happening 100x faster than it has for millions of years, then you have a problem. Previous rapid changes in OA (ie the PETM) nearly wiped out corals.
Like just about everything with climate change, if it happens slowly then it doesnt cause a problem. It is the rate of change, especially compared to rate at which adaption can occur, that it is the issue.
Russ R @1:
1) When CO2 is dissolved in sea water, its tendency to acidify the water is buffered by other chemical compounds, including Aragonite (CaCO3). Those compounds are replenished by weathering, which increases with increased temperature. For most periods in the past the two are balanced.
If you have a very rapid increase in CO2, the buffering agents will be exhausted allowing a far greater drop in ocean pH (ocean acidification). An equivalent increase over a long period will allow an increase in weathering that limits the fall in pH. Therefore ocean pH in times of high CO2 concentrations in the past will have been much higher than they will be if we achieve the same levels over the next century.
Further, with slow increase of atmospheric CO2, there is a build up of aragonite. That is, while ocean pH is lower, aragonite concentrations are also higher which makes shell building easier at a given pH level. In constrast, with a very rapid rise in CO2 concentration, both pH and aragonite concentrations fall, with both effects making shell building harder.
Further reading.
2) The organisms and species alive today are not the same as their ancestors in the distant past. They have evolved for different conditions, including for higher ocean pH. Potentially, given sufficient energy, mechanisms existed to build shells in the past with lower pH, but those mechanism would have been energetically expensive, and cannot be presumed to have been preserved in situations with high pH.
Assuming that because ancesttors of modern species were capable of forming shells survived with low pH in the past, their descendants can do so today is like assuming that because humans are descedant from brachiating apes, we can swing through the trees in a jungle as rapidly as a chimpanzee or orangoutang. It simply does not follow.
Potentially, the ability to construct shells with low pH could evolve again. Evolution, however, is a process that occures over tens of thousands of years - far to slow to help species at risk over the next couple of centuries.
Further to my post @3, here are pictures of reef growth near a volcanic vent in New Guinea, along with a map of local ocean pH:
(Source)
Frame B corresponds to the ocean pH projected by the end of this century with BAU.
The volcanic vents have existed long enough that any preexisting adaptive mechanism would have been able to kick in. Never-the-less, there is a substantial reduction in coral diversity with low pH, and an almost complete absence of branching corals. The idea that vulnerable organisms will simply adapt to low pH is already refuted in reality by their failure to do so in situations of already existing low pH.
The science is not yet definitive, but there have been some big die-offs of scallops and oysters offshore British Columbia, warming and acidifying waters appear to be prime suspects.
Mystery surrounds massive die-off of oysters and scallops off B.C. coast
Russ R, Doug Mackie wrote an excellent series of blog posts called OA is not OK that is well worth reading to understand the basics (there are 20 posts, which show there is quite a lot you need to know and "simple chemistry" isn't quite enough).
Good responses all. Highly informative.
Three followup questions, two scientific and one semantic.
Russ R., regarding your question #3: No, "acidification" is not misleading. It was a common term long before the human cause of ocean acidification was a hot topic. Saying "it is acidifying" instead of saying "it is becoming less alkaline" is no more misleading than saying "it is warming" instead of "it is becoming less cold."
Also, please do follow Dikran's advice about the background reading.
Russ R. - Regarding past episodes of acidification, Hönisch et al 2012, The Geological Record of Ocean Acidification, is a recent and relevant paper. They examine among other data boron isotope composition for pH, calcium-to-trace element ratios for ambient CO2, and alkenone carbon isotope composition for aqueous CO2.
In table S1 of the paper (supplemental data) they compare these past episodes to the present, and find the only really comparable episode is the Paleocene–Eocene Thermal Maximum (PETM). The PETM notably had a mass extinction of shell-forming foraminifera. This recent work with direct proxies for pH and CO2 changes agrees with previous research on the PETM and its similarities to the present.
Regarding your "hard to believe" question (Argument from Incredulity?) on pH swings, seasonal variations are short term and can be managed by many organisms, while longer term average pH changes induce energy costs (energy of fixation in shells) and the lifespan availability of aragonite and calcite needed to build shells. And yes, there are nonlinear thresholds (Ries et al 2010) for many organisms.
Finally, the correct and proper chemical terminology for lowering pH is indeed "acidification" - semantic arguments in that regard don't affect changing H3O+ concentrations, and are irrelevant red herrings. If you start at the South Pole and travel a few hundred km in any direction, you are moving north (northification?) despite still being in the Southern Hemisphere, and the weather will be correspondingly different there.
Ocean acidification imperils Alaska’s fishing-dependent economy, says a new study funded by the National Oceanic and Atmospheric Administration.
For details, see:
Southeast, southwest Alaska communities at highest risk from ocean acidification, study says by Yereth Rosen, Alaskan Disptach News, July 29, 2014
A side note on terminology.
"Acidic" describes chemicals that are currently of low pH, defined as below 7.0. It is an adjective modifying the noun, the chemical. "Acidification" describes shifting from current pH to a lower one, the change thereof, and is an adverb for changing pH, modifying the implicit verb and indicating direction.
Different parts of speech entirely. Yes, the oceans are currently about pH 8.1, alkaline. Which is roughly a 30% change in H+ ion concentration since pre-industrial levels (Jacobson 2005), an acidification. People who object to properly discussing the direction of that change are missing some essentials of grammar.
Russ R asked: "Since the ocean pH at >8 isn't in the "acidic" range of the scale, and since pH isn't even the primary issue here, isn't the term "Ocean Acidification" more than a bit misleading?"
As Tom & KR explained, 'acidification' is the correct term. It's the claims to the contrary which are, "more than a bit misleasing". Whereever you are getting this stuff... skepticism ought to impel you to start asking why they are feeding you nonsense.
We can cope with a 10 degree difference in temperature between spring and summer but we surely cant cope with a 10 degree change in average temperature. Ditto to seasonal change in pH. Most shellfish also have a highly seasonal pattern to shell growth as well.
Russ R @7, as one of the original respondents to your first post, I trust the moderators will not see me as "piling on".
1 a) In fact it is very difficult to get good proxies with millenial resolution in the distant past, let alone decadal. However, your original question was focussed on episodes of sustained depressed ocean pH. During those sustained periods there were shell building organisms present (made possible by buffering by increased weathering). You have now switched your question, and you are no longer entitled to your assumption of onging existence of shell building organisms.
For example, there have been sustained periods in the past with a noted absence of reef building organisms in the ocean:
(Source)
Several of these events are associated with periods of rapid increase in CO2 levels in geological terms, on which more below.
The question will obviously arise, if the corals go extinct, how do they come back in 5 million years. The obvious answer is that while members of the same family, or order of corals survive, members of the same genus and species do not. In particular, what probably has occured is that either a related soft coral has evolved to occupy the vacated niche; or a surviving species or small number of species of hard corals have successfully made the transition to a soft coral niche, and then reevolved the reef forming habit once conditions were more suitable.
We can be sure that some measure of evolution was involved because of the 5 million year gaps. Had a small number of hard corals retained the hard coral habit in refugia (isolated areas were pH is sustained and higher levels by local geochemistry), restoration would have been almost instantaneious in geological terms (100,000 years or less).
So, on the plus side, rapid ocean acidification will likely only eliminate coral reefs for the next five million years. Is that really any different from eliminating them forever in human terms? And once the five million years are up, related corals may take up the reef forming habit. Or perhaps not. After all, to previous forms of corals (Rugosa and Tabulata) did not come back after the end Permian extinction, being replaced by an entirely different form of coral.
1 b) It is highly unlikely that many past excursions in CO2 concentration have been as rapid as the current excursion. Among the most rapid (geologically speaking) causes of increased are large igneous provinces such as the deccan traps, of which wikipedia says:
The CO2 content of flood basalts as a proportion of mass is well known. So also are the timings of eruptions in flood basalts (igneous rock being the easiest to date). That has allowed Self et al (2006) to estimate the rate CO2 emissions as a result of the formation of the Deccan traps:
Even assuming the entire Deccan traps were formed over the 33 million years of peak erruption, that amounts to an annual average emission rate equivalent to of 0.03 ppmv. Human emissions are currently 100 times that rate.
If even the formation of the Deccan traps cannot hope to match current human emission rates, and hence current rates of change in ocean pH, rates of change in ocean pH equivalent to the modern must be rare to non-existent in the past. It is possible that such rates have been matched by either large scale clathrate release (PETM) or large igneous provinces igniting larger reservoirs of fossil fuels (suggested for the end Permian extinction), but all such potential instances are associated with large scale extinction events, particularly among animal types known to be vulnerable to ocean acidification.
2 a) You cite pH values for water intake at Monterey bay. In enclosed waters such as bays, pH values are often far lower than in the open ocean, and are far more variable. A more appropriate comparison (because not all threatened species live in bays) is with monthly variations in open ocean pH:
There you see a peak intra-annual increase of just 0.07 pH over four months, and peak declines of slighty less magnitude. That is, the peak monthly change in open ocean pH is less than the change in open ocean pH already brought about by anthropogenic emissions of CO2.
2 b) scaddenp @13 correctly notes that changes in seasonal values do not have the same impact as changes in annual averages. Specifically, molluscs in Monterey bay, for example, may have an annual cycle in which they build up shell thickness during periods of high pH, can loose shell thickness during periods of low pH. A general lowering of pH may then restrict the build up in one season and increase the decline in shell thickness in the other - weakening shells overall and (if sustained) eventually eliminating them.
You can reasonably point out that that is a hypothetical mechanism, but what you cannot reasonably do is ignore the numerous examples of recorded shell loss, or depleted reef construction rates, and of inability of reefs to colonized otherwise suitable areas with low pH in the wild. The adverse impacts of low pH on a number of marine animals is not hypothetical. It is observed.
The article contains the comment 'Unlike marine pollution and overfishing, which require multifaceted solutions, ocean acidification has only one primary cause: excess carbon dioxide in the atmosphere. It therefore has an obvious solution: limit carbon emissions.' There is no possible solution in the time frame relevent to civilization. The current carbon dioxide concentration level is 400 ppm, well above the preindustrial level, and this excess carbon dioxide is causing the ocean acidification. Limiting future (rates of) carbom dioxide emissions will only slow down the the rate of increase of the concentration level, so the rate of ocean acidification.
I hope someone can answer this question about ocean acidification. An oceanographer/ecologist that visited our university claimed that ocean ph around the coasts is mostly controlled by runoff and ocean acidification wont have a strong effect. The only places ocean acidification will have strong effects is away from coasts and where deep ocean upwelling occurs next to coasts. Since most corals are in coastal waters ocean acidification is not an important problem for corals.
also
Most of the ocean fish biomass is in middle deep living fish that can't be caught in nets (i looked it up its true), and this ecology has been basically untouched by humans. Since the largest amount of ocean fish biomass is untouched the oceans are better off than we think and the fish we do take are such a small part of the ecosystem that its not a real problem. I guess my answer is just because its the largest biomass does not mean its the only important thing about the ocean but maybe someone can add to this.
(Rob P) - Why would an oceanographer/ecologist speak at your university on a topic they clearly know very little about?
The geological record indicates that ocean acidification was a kill mechanism in 3 of the 5 major extinction events, and contrary to popular belief, reef-building coral of today are not the same ones which lived in the oceans hundreds of millions of years ago - those ancient coral became extinct when the tropical surface ocean became too warm and too corrosive. This is why there are a number of 'reef gaps' in the fossil record.
The oceans are now acidifying faster than at any time in the last 300 million years and, as might be expected, coral worldwide are in rapid decline (not only due to acidification though).
It would be nice to be optimistic about all of this, but the evidence paints a rather gloomy future.
@ Jim #16: The assertions contained in your first paragraph do not seem to square with what's happening up and down the Pacific Coast, from California to British Columbia to Alaska.
Read more: http://www.smh.com.au/environment/climate-change/intensifying-ocean-acidity-from-carbon-emissions-hitting-pacific-shellfish-industry-20140731-zyrg6.html#ixzz393thfreB
@ Jim #16: Your second paragraph includes the following:
Most of the ocean fish biomass is in middle deep living fish that can't be caught in nets (i looked it up its true), and this ecology has been basically untouched by humans.
Please provide the source of your information.
John H.,
He's probably referring to this Nature communications article by Irigoien et al from February of this year. It's an attempt to estimate mesopelagic fish biomass from acoustic data rather than trawls, which are presumed to be biased. These fish are presumed to be so abundant because they feed low down on the food chain (being small) and are not preyed upon very heavily because of their nightly migration from the deep. I'm not sure how they ground truth the acoustic scattering data since all other methods are considered biased.
In any case, they are a giant red herring (so to speak). We don't know if the mesopelagic fish are "untouched." It's possible humans have had a positive impact on these organisms by removing large pelagic predators. That could in turn have effects on the extent and intensity of oxygen minumum zones at depth, through respiration. It's also possible there are negative consequences of ocean acidification on the food base of these organisms, which are organisms in the surface layers to which they migrate daily. Then there is also the general increase in gelinous zooplankton in many region sof the world.
Basically, the biology of the "mesopelagic twlight zone," as it's called, is all up for grabs and subject of an extensive research effort right now. To use that lack of knowledge as proof that all is alright is profoundly silly.
jim @16,
I'm not sure what your question is, but the basic story is that it depends on where you are.
Variations in pH in coastal regions reflect the balance of respiration in plankton and in deep sediments (which produces CO2), photosynthesis (which consumes CO2), upwelling of deep water (which has lots of CO2) and inputs of rivers (which often also have a fair amount of CO2 as well as other inorganic and organic acids). As a result, pH can vary a fair amount (easily 0.5 units) over time scales of days in coastal systems with variable upwelling, high algal growth, shallow water columns and large rivers. It's also true that organisms growing in such environments are often capable of handling, and even preferring, variations in pH that result, while open ocean species are typically less equipped to handle such variation.
However, its also true that progressive acidification combined with such variation makes it more likely for pH to drop to levels that may be outside the typical environmental conditions to which these organisms are adapted. The parallel with how gradual atmospheric warming combines with weather variability to produce a large increase in the probability of damaging extreme temperature events is obvious. We don't know in many cases what the critical pH thresholds are for many species, or how long pH must stay below them to have a significant impact.
Also, as indicated by the article to which John Hartz points, atmospheric CO2 in the past influences the pH of deep water brought to the surface now, and current CO2 will lead to lower pH in such water in the future in regions exposed to upwelling, so the the effects of upwelling and atmospheric CO2 on acidification are not really independent, just lagged in time.
The generalization about coral reefs is completely off the mark. There is a reason we don't see coral reefs off of heavily populated temperate coasts. Reef building corals require warm waters with relatively high pH that are not subject to upwelling of deep water. They also do not like the extra nutrients and sediments that are brought in by rivers or are introduced as a result of human activity. Because they prefer those factors be absent, and because they need relatively high pH to build calcium carbonate shells, corals in particular are likely to be directly affected by ocean acidification.
@ John H Thanks for the article it is helpful. However, the upwelling along the pacific coast supports the case was arguing. "Although seasonal upwelling of the undersaturated waters onto the shelf is a natural phenomenon in this region, the ocean uptake of anthropogenic CO2 has increased the areal extent of the affected area." http://www.ncbi.nlm.nih.gov/pubmed/18497259
The acidified ocean off the pacific coast is natural unique feature to the area that CO2 increase has enhanced, not a general feature coastal waters.
from your link: "On the East Coast, instead of upwelling, acidification is a result of nutrification - adding nutrients like agricultural waste, fertilizers and waste water treatment facilities. The Chesapeake Bay, which receives runoff from one of the most densely-populated watersheds in the United States, is acidifying three times faster than the rest of the world's oceans. Long Island Sound, Narragansett Bay and the Gulf of Mexico are all showing signs of rapid acidification."
Again this seems to support the case that it is coastal features that are important to the ocean acidification problem. In the east coast the acidification is due to nutrient run off and not atmospheric CO2.
I suppose my question is where will ocean acidification from atmospheric CO2 be a problem? As far as i can tell it is only in specific locations where that deep ocean water is brought near the coasts. I would still like to have a better idea where this process is occurring.
@ Stephan that was the study I saw. I appreciate the answer.
@ Stephan just saw your second post. Thats helpful. I just found this feely paper that i am reading that seems to answer some of where these effects are occurring. however its a broad simulation not detailed. http://www.tos.org/oceanography/archive/22-4_feely.pdf
From figure 7 perhaps the greatest concern is arctic waters. This is a complex issue.
Im still not sure how to refute my initial statement that ocean acidification due to CO2 is primarily a problem in the open ocean and not near coastal waters.
I guess my answer will be that more needs to be done on specific areas. Also, it's wrong to imply that most of the ocean community we are concerned with is dominated by the effects of runoff.
What i can find on corals seems to indicate it is nutrient run off, invasive species and warming water that is a problem. http://www.pnas.org/content/109/44/17995.full
(Rob P) - Eutrophication (excess nutrient run-off) of coastal waters simply accelerates the acidification process, it doesn't mean that CO2 is somehow magically not dissolving into coastal seawater.
Ocean acidification is indeed a global phenomenon, but some areas - such as the polar seas where colder water is able to absorb more carbon dioxide - are more susceptible. The same applies to polar land regions which are now undergoing thaw.
All that extra organic material being flushed into the ocean is broken down by bacteria and releases CO2 into the water column - thus accelerating the acidification process. This is soon going to be a huge problem in the Arctic.
jim,
De'ath et al did not consider acidification effects on reefs, and they explicitly state that they probably underestimate coral reef decline on the Great Barrier Reef as a result. Just because a study only focusses on some factors affecting coral reefs does not mean that it concludes others are not important.
Also, it's important to realize that while human nutrient pollution, resource harvesting and land use have been fairly advanced for some time, ocean acidification is in many ways only beginning. Negative effects on corals are likely, and sometimes observed, but many won't be fully manifest for some time, prehaps until pH in these regions approaches the saturation point for calcite/aragonite. Unlike those other problems, which we largely dealt with post-hoc, we are a touch ahead of the curve in assessing the impacts of ocean acidification, even if we're not necessarily coming up with solutions.
The fact that waters off the east coast of the US are acidifying faster than elsewhere means only that factors other than the increase in atmospheric CO2 also influence local patterns in pH, as I pointed out, and it is important to understand those other factors. However, it also means that fully one third of the increase in LIS and Chesapeake Bay ecosystems is directly attributable to increased CO2 in the atmosphere. This effect exacerbates the effect of the other factors on pH, and it will only increase in importance in the future. It makes it much more likely that critical thresholds will be crossed under extreme conditions. Future increases in CO2 may also render attempts at remediation of pH through pollution control unworkable.
In short, I'm not sure it makes sense from a risk avodance point of view to think about ocean acidification as a process that is important in one place and not another. Yes, organisms adapted to more constant conditions are likely to be more vulnerable, but so may be organisms in cold areas that are already acidic, or organisms at the northern ends of their ranges who may be near thresholds. Moreover, the combination of local variation with a longterm trend in pH could mean that critical thresholds could be crossed sooner in coastal systems under extreme conditions. Since we depend heavily on the living resources of coastal ecosystems, it would be unwise to deny that risk until we know more.
@ Rob P Thank you for the comments, very helpful. The visiting scholar was a seagrass expert and well regarded. I didn't want to put his name out because I might be misrepresenting his arguments. The context was in a closed student question and answer session and he was giving many reasons why he was optimistic about global warming. He gave the two reasons above plus a bunch of evidence that I can't remember the reference for as to why he thought there was a lot of adaptation potential in the ocean ecosystems. I would call him a climate change optimist. He seems to think that nutrient pollution is a huge problem while climate change CO2 is a minor problem. I dont want to attribute anything I just said to him because I am sure he would be much more nuanced. However that was my take away.
Here is an article in his own words:
http://theconversation.com/is-the-ocean-broken-19453
There is obviously a lot of unknowns about how the ocean ecology will respond in the future. It seems in the dearth of evidence there is room for both optimistic and pessimistic arguments. I honestly dont know how to approach this except to keep an open mind and ask for more research.
(Rob P) - Fair enough Jim - something nuanced can certainly get lost in translation. Don't get me wrong, as far as ocean acidification is concerned we are not locked into an extinction scenario, but we had better start taking it very seriously very soon.
jim@16, and following comment:
1) Here is the marine ecologists discussion of this issue in his own words:
Fish in the twilight cast new light on ocean ecosystem
2) He was clearly wrong if he implied that mesopelagic fish are not subject to fishing. In particular the Patagonian and Antarctic toothfish are mesopelogic predators that have been subject to extensive fishing since 1996. In 1999-2000, 26,000 tonnes of tooth fish were caught in southern waters. That represents, perhaps, just 0.1% of the total mesopelagic fish mass (using the upper estimate from the article linked above, and allowing for the extension to 70N-70S discussed the paper refferenced therein).
Toothfish are primarilly caught by longlines. That means the methods of evasion discussed in the article are not relevant to them. In fact, the method rather works in the reverse, with keen eyesight and the ability to swim rapidly extending their area of vulnerability to a longline.
3) The majority of mesopelagic fish feed on pykoplankton (PP), and hence are presumably not liable to be caught by long line fishing. However, predatory PP fish are. In addition to tooth fish, Duarte mentions two other predators of mesopelagic fish (Tuna and Swordfish) that are currently being actively fished, and indeed overfished. He has described current fishing of Tuna as so far from sustainable that it amounts to a "war on tuna".
The first lesson in ecology anybody learns is the effect of removing a top predator from an ecosystem. The result is a population boom in the predators prey. The prey then over feeds on its food source, leeding to a collapse of population of the foodsource, followed by that of the prey with the result of a series of osscilations in populations that are chaotic, and hence not predictable. Given that, and the known overfishing of predators of mesopelogic fish, a one time sample of mesopelagic fish cannot be assumed to represent a stable population - particularly when that assumption leads to a further assumption of much greater then expected efficiency in grazing on primary production.
4) Despite that, this is good news in terms of long term ocean health, if irrelevant for the human centered question of whether or not we are sustainably fishing the planet. (It is irrelevant to question of whether we are fishing at a rate that will allow us to catch fish at the same rate sustainably into the future if we suddenly discover a large population of uncatchable fish.)
Jim @24
Re optimism...
There is no need to protect Carlos Duarte...he is out in public for a reason.
Part of Carlos's "optimism" regarding CO2 may come from the fact that he is, at heart, a seagrass ecologist. Seagrasses have been shown to increase photosynthesis in response to increasing CO2, presumably because they are more strongly limited by diffusion of CO2 than other nutrients and light. Carlos has coauthored papers on the role of seagrass beds as carbon sinks, and has suggested using them in CO2 remediation. Seagrass beds can experience wide ranges in pH and CO2on even a daily cycle, not to mention over seasonal scales.
He's also orginally a strict empiricist from the McGill school who places his faith on hard data, statistical analyses and successful prediction, and who reflexively questions claims he feels have an emotional basis. This is a very admirable quality. However, one can make the case that this strict empiricism — the need to see a significant effect before admitting a phenomenon is possible — may be a little blind to anticipating problems. In statistical parlance, it makes you subject to Type I errors.
In the larger picture, though, I think that Duarte is really trying to counteract despair and the paralysis it produces. Some people feel strongly about the ocean as an untouched frontier. These people often find it hard to accept that there really is no place on earth that has not been affected by humans. The extent of our influence can lead them to despair of hope. Carlos hopes to counteract this by grounding the discussion so people can reframe how they think about the ocean and try to make realistic, focussed choices, rather than simply rending their hair and calling names, which of course only alienates others.
I agree with him in that I would not decribe the ocean as "broken" or "dead," terms I sometimes hear in the public discourse and in the classroom. Those terms are too broad and emotional, and they don't suggest a way forward, only a way to feel. Knowing him, though, I'm also pretty sure he would not say the ocean is in great shape and that there is no chance it will get substantially worse. It definitely could (and has) in the absence of proper management. (Luckily...good management has often resulted in good results!) In that light I'd describe him as a "climate change optimist" only in comparison to those that despair — but that is a pretty low bar.
Where he is undoubtedly an optimist is that he also thinks we can repair/avoid problems in the ocean and maintain our standard of living IF we take the right actions, but those actions must be grounded in a real appreciation how the ocean behaves, not in ideals about how it should be, separate from humans. He also is optimistic that communication of scientific findings can be done well and in a way that influences peoples actions.
Really, that describes the kind of optimism that everybody on this board displays if you think about it. At this point, it's an attitude of necessity.
Tom C @23
Re nets and fish
I'm pretty sure that Duarte, in those articles to which Jim and you link, is talking about lanternfish, mostly, as they are the highly sensitive small bioluminescent zooplanktivorous fish which make up much of the biomass in the mesopelagic. They aren't caught in typical purse seines or trawls typical of commercial fisheries, and are too small for long -lines. So they aren't of interest commercially.
They can be caught sometimes in zooplankton nets with 0.2 - 1mm mesh that are drawn on frames a couple meters square or smaller through the water. Such nets are mostly used by scientists and produce a pretty large pressure wave in front of them that sensitive mobile species can sense and avoid. Plus, they are dragged behind a boat which also scares mobile organisms ahead of the net. the point of the article Jim links to is that these nets to a bad job of catching them, which I believe.
I will say that it is a little strange to say the ocean is "healthier" than you thought previously simply because you found a lot of biomass that you didn't know was there before, as he appears to in the article you link to. The very point of the research is that we don't know how the status of mesopelagic fish has changed...or even what such a change would mean...since we know so little about it.
I guess his idea is that biomass is biomass and that a large biomass of fish that hasn't been harvested means less biomass lost to fishing. But that will be little solace to fisherman with empty purse seines and long lines, not to mention consumers paying lots for fish! It's kind of akin to those who point to life prospering in warm acidic oceans of the past, ignoring the fact that the life that prospered then was basically bacteria and of little relevance to our future in the face of climate change.
Then again, I see that the article quoting him characterizes a Mola mola (ocean sunfish) as a mesopelagic fish, which is crazy, so maybe they misunderstood him!
Key words here are :Evolved over 1/2 billion years
They evolved and were able to evolve during climate changes over very long periods of time. Climate change like we are seeing today used to take thousands s to 10's of thousands of years allowing many forms of life to evolve with the change. Currently that same change in climate is taking place in a highly compressed 100 year timeline and nothing can evolve that fast with the exception of a few viruses and bacteria ....
The speed of climate change effects lifeforms as much or more than the amount of total change.