Climate Change Cluedo: Anthropogenic CO2
Posted on 25 July 2012 by Tom Curtis
Anthropogenic CO2?
The human-caused origin (anthropogenic) of the measured increase in atmospheric concentrations of CO2 is a cornerstone of predictions of future temperature rises. As such, it has come under frequent attack by people who challenge the science of global warming. One thing noteworthy about those attacks is that the full range of evidence supporting the anthropogenic nature of the CO2 increase seems to slip from sight. So what is the full range of supporting evidence? There are ten main lines of evidence to be considered:
- The start of the growth in CO2 concentration coincides with the start of the industrial revolution, hence anthropogenic;
- Increase in CO2 concentration over the long term almost exactly correlates with cumulative anthropogenic emissions, hence anthropogenic;
- Annual CO2 concentration growth is less than Annual CO2 emissions, hence anthropogenic;
- Declining C14 ratio indicates the source is very old, hence fossil fuel or volcanic (ie, not oceanic outgassing or a recent biological source);
- Declining C13 ratio indicates a biological source, hence not volcanic;
- Declining O2 concentration indicate combustion, hence not volcanic;
- Partial pressure of CO2 in the ocean is increasing, hence not oceanic outgassing;
- Measured CO2 emissions from all (surface and beneath the sea) volcanoes are one-hundredth of anthropogenic CO2 emissions; hence not volcanic;
- Known changes in biomass too small by a factor of 10, hence not deforestation; and
- Known changes of CO2 concentration with temperature are too small by a factor of 10, hence not ocean outgassing.
Annual emissions of CO2 by human use of fossil fuels rose from 3 million tonnes of Carbon (11 million tonnes of CO2) in 1751 to 54 million tonnes of Carbon (198 million tonnes of CO2) in 1850. After that fossil fuel use rose sharply so that by 2008, annual emissions (including from cement manufacture) had risen to 8749 million tonnes of Carbon (32 billion tonnes of CO2). The rise in the atmospheric concentration of CO2 also began around 1750 and has followed the emissions up. This extraordinary coincidence strongly suggests that anthropogenic emissions are the cause of the rise in CO2 concentration.
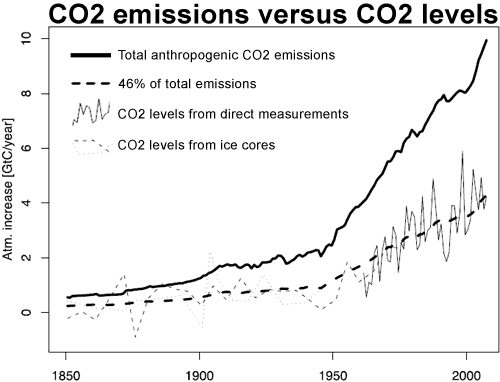
The increase in CO2 concentration over the long term (1850-2005) almost exactly correlates (corr.: 0.997; R^2: 0.993) with cumulative anthropogenic emissions from all sources including Land Use Change (LUC). The close correlation has continued in recent times, with a correlation of 0.9995 when compared to the Mauna Loa record (r^2: 0.999). So exact a correlation would be extraordinary if anthropogenic emissions were not the cause of the increase in CO2 concentration.
Over the course of the twentieth century, the increase in CO2 in the atmosphere measured in tonnes has been less than anthropogenic emissions in every year, and has averaged only 44% of anthropogenic emissions over the period from 1850-2005. Indeed, growth in atmospheric emissions probably has not exceeded anthropogenic emissions since the early 1880s, approximately the time anthropogenic emissions reached the equivalent of 0.45 ppmv of atmospheric concentration. It is interesting to note that the airbourne fraction, ie, the atmospheric increase divided by total emissions, has increased slightly in recent times. This means that natural carbon reservoirs have acted as a net sink over the course of the 20th century, and strongly indicates that the source of the increase in CO2 concentration is anthropogenic.
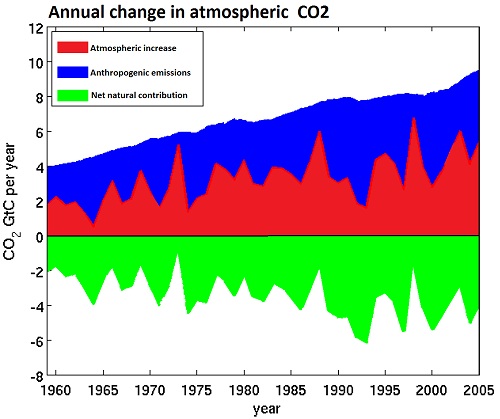
(Adapted from Cawley, 2011; data from CDIAC)
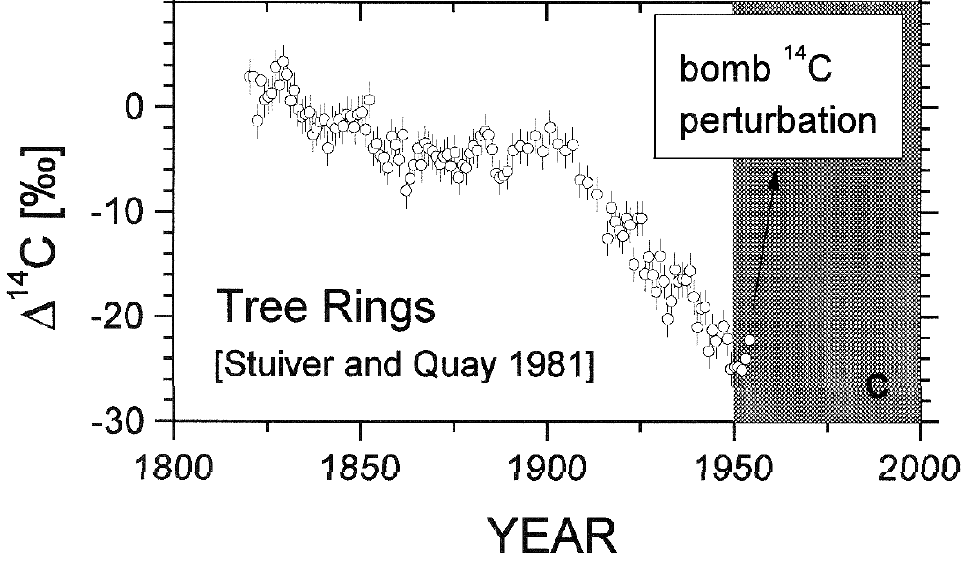
Carbon 14 is formed in the atmosphere by collisions between cosmic rays and Nitrogen. It has a very short half life (5,730 years), but atmospheric C14 is continuously replenished, maintaining a near constant concentration. Buried C14 is not replenished, however. As a result, whether from volcanoes or fossil fuels, CO2 from long-buried sources has effectively no C14. The addition of large quantities of CO2 from a long-buried source to the atmosphere will result in a significant decline in C14 concentration in the atmosphere, which is what we see. More recent, high precision measurements show the decline in C14 continued after the end of atmospheric nuclear testing.This is strong evidence that the source of the increased concentration of CO2 is fossil carbon, either from volcanoes or from fossil fuels.
Carbon has two stable varieties (isotopes), C12 an C13. Because C13 has an extra neutron, it is heavier. In photosynthesis, most plants find it easier to take up the lighter C12, and do so at a higher rate than they take up C13, with the result that carbon compounds formed from the products of photosynthesis, including plants, animals and fossil fuels have a lower C13 to C12 ratio than does the atmosphere. Introducing a large quantity of CO2 derived from photosynthesis would cause the C13/C12 ratio to decline. In contrast, CO2 introduced from volcanoes or from outgassing from the ocean would not significantly affect the C13/C12 ratio. In fact the global C13/C12 ratio has declined, which is very strong evidence the source of the CO2 increase has was C12 enriched, ie, derived from photosynthesis. Therefore it is very strong evidence that it comes from the biosphere or fossil fuels, rather than from volcanoes or oceanic outgassing.
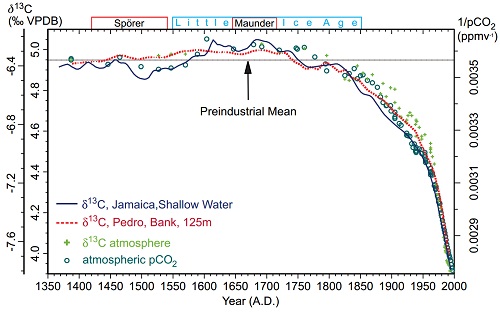
6) Declining oxygen concentration
Because the change in solubility of O2 in water with change in temperature significantly differs from that of CO2, the change in O2 concentration is not affected by other possible CO2 sinks. That means the decline in CO2 concentration means any large unknown natural sources of CO2 must not come from a source of combustion but must come from a low C14 source generated by photosynthesis. These facts together almost completely preclude the existence of such putative natural sources. Because of the importance of the O2 decline, it is worthwhile looking at the chart below from the IPCC TAR which shows it:
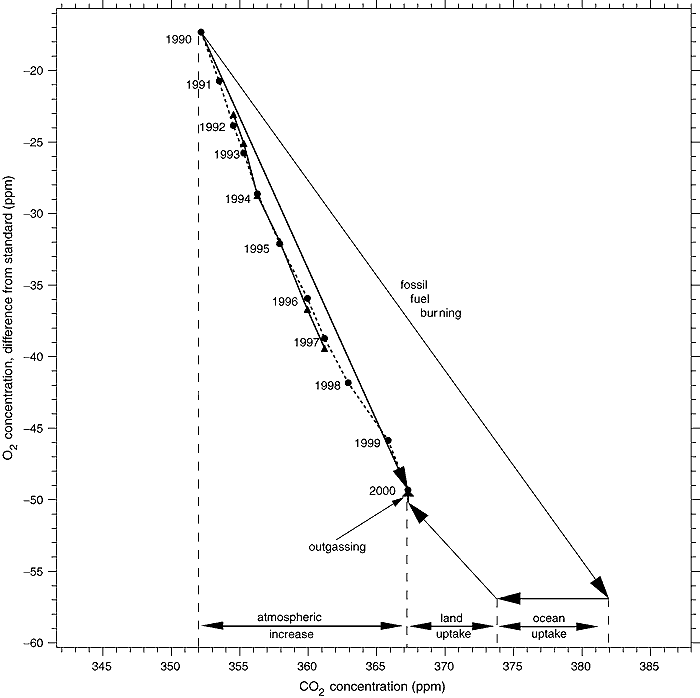
(Source)
The observed decline in O2 is straightforward. The diagonal arrow from the start point marked "fossil fuel burning" represents the expected change in CO2 and O2 concentrations from known fossil fuel consumption. The arrow marked "ocean uptake" represents the uptake of CO2 by the ocean, which does not affect the O2 level. The arrow marked "land uptake" is the uptake of CO2 and release of O2 by photosynthesis, which also decreases the CO2 concentration and increases the O2 concentration. Finally, the small arrow marked "outgassing" represents outgassing of O2 from the ocean, which does not affect CO2 concentration. That outgassing is partly the result of a warming ocean, and partly a result of the very slight decrease in the partial pressure of oxygen in the atmosphere. These factors are reasonably, but not exactly known. It is important to note that because the fall in O2 concentration is significantly less than that predicted from known combustion of fossil fuels, the uptake of CO2 by photosynthesis must exceed the combustion or decay of modern organic material from either anthropogenic (Land Use Changes) or natural sources.
7) Increasing CO2 concentrations in the ocean
Simultaneously with the rise in the concentration of CO2 in the atmosphere, the concentration of CO2 (and its equilibrium products) in the ocean has been increasing. The rise in CO2 in the ocean is referred to as an increase in the "partial pressure" of CO2, and results in a simultaneous decline in the partial pressure of Hydrogen (pH), ie, an increase in the ocean's acidity. If the amount of CO2 in the ocean was falling, the partial pressure of CO2 would be falling, and the pH rising. This is very strong evidence that oceanic outgassing is not the cause of the rise in atmospheric CO2.
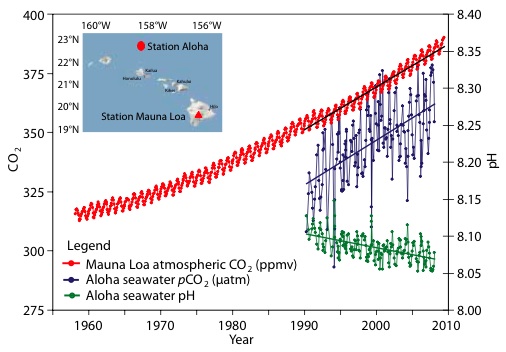
(Source )
8) Measured volcanic emissions
Scientists have used a variety of methods to determine the CO2 emissions from volcanoes. A common method is to use a tracer gas, ie, a gas emitted from volcanoes but which does not stay in the atmosphere for long. Determining the emissions rates of the tracer gas from volcanoes, together with the concentration of those gases in the atmosphere allows the overall level of volcanic activity to be measured. Once that is measured, measurements determining average rates of CO2 emissions for a given amount of activity can be used to determine the global CO2 emissions from volcanoes. Other techniques are used to measure CO2 emissions from volcanoes, mid-ocean ridges and subduction zones under the sea. The emissions, from all volcanoes, both on land and under sea, are about one hundredth of anthropogenic emissions. While there may be some error in the estimates, it is unlikely that the error would be large enough for volcanoes to be emitting a sizable fraction of anthropogenic emissions. That strongly suggests volcanic emissions are not the source of the increased CO2 concentration.
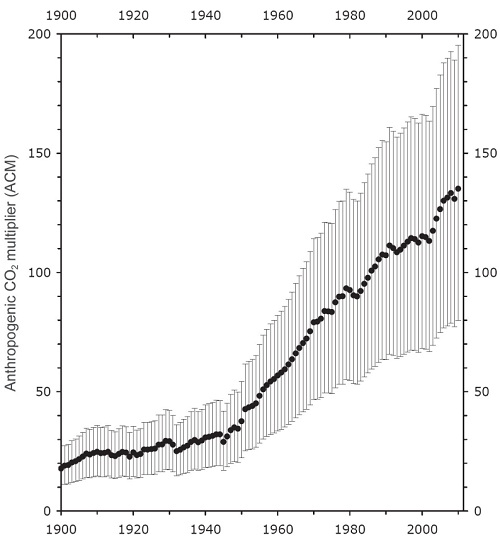
(Ratio of anthropogenic to volcanic CO2 emissions; source)
Anthropogenic Emissions from Land Use change and deforestation represent 10% of all human emissions (0.9 PgC of 10 PgC). Over the last century, human-caused deforestation and other land use changes have been by far the largest cause of change in land cover, and hence natural changes cannot be significantly larger than that.(Source) Indeed, as discussed regarding the declining oxygen concentration, that decline together with the land uptake shows that the biosphere is a net sink for CO2.
10) Past changes in CO2 concentration
During past "ice ages" (glacials) CO2 concentrations have correlated with temperature, with approximately a 22 ppmv increase in CO2 for every 1 degree increase in temperature. In more recent historical times, there was an increase in CO2 concentration during the Medieval Warm Period (MWP) relative to the Little Ice Age (LIA) of about 10 ppmv for approximately a 1 degree C change in temperature. Given that global temperatures have increased by 0.7 C since 1850, we would expect an increase in CO2 concentration of between 7 and 15 ppmv based on historical precedents. That CO2 concentrations have increased by approximately 110 ppmv over that period is very strong evidence that the source of the increase was not outgassing from the ocean.
(Source)
Playing Climate Change Cluedo
As a child I enjoyed playing Cluedo (Clue in the US market). I soon learned you discovered more from the questions people did not respond to than from those that they did, and developed a matrix from which to plot responses and non-responses. Filling in the matrix soon honed in on the correct answer, who killed whom, with what and where. Science is sometimes like that. The lines of evidence are the questions we put, and if we plot out our matrix, it quickly becomes clear that it is the humans who have caused the rise in CO2 levels, by burning fossil fuels in the twentieth century. Every other hypothesis makes a host of predictions that do not pass the test of the evidence.
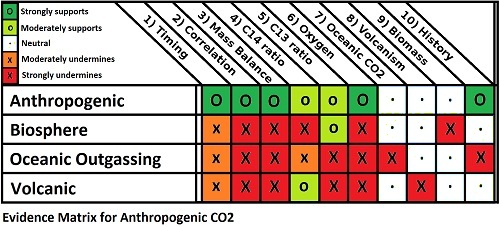
Correction: The figures in section (2) have been corrected as of 12:45 PM, 29/07/2012. The nature and reason for the correction explained in my comment of 12:40 PM of that date.
The figure for the airborne fraction in section 3 was corrected at 8:54 AM, 18 th Feb, 2013.































 Arguments
Arguments


























 0
0  0
0






Comments