The human fingerprint in global warming
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
Multiple sets of independent observations find a human fingerprint on climate change. |
|||||||
Climate Myth...
It's not us
'What do the skeptics believe? First, they concur with the believers that the Earth has been warming since the end of a Little Ice Age around 1850. The cause of this warming is the question. Believers think the warming is man-made, while the skeptics believe the warming is natural and contributions from man are minimal and certainly not potentially catastrophic à la Al Gore.' (Neil Frank)
At-a-glance
Since the pre-industrial era, we have burned ever-increasing amounts of fossil fuels. This we can see for ourselves, often just by looking out of the window or hear, by listening to the flow of traffic. The sights and sounds of fossil fuel burning, constant, perpetual.
We cannot see or taste carbon dioxide, one of the two main products of that combustion, the other being water vapour. But it's constantly heading up into our atmosphere – in 2021 alone the figure for such emissions, according to the International Energy Agency, was 36,300,000,000 tonnes of the stuff. Just imagine for a moment that amount of sand piled up in a heap!
Once up there, one key difference between carbon dioxide and water vapour becomes critically important. Water vapour condenses to form clouds and freezes to form ice crystals. Rain, hail, sleet and snow all fall from clouds – it's a constant, ongoing cyclic process, even though different places see much more – or much less precipitation.
Carbon dioxide has no liquid state at the pressures encountered in Earth's atmosphere. Instead, at normal atmospheric pressures, it sublimes from solid to gas at −78 °C. Solid carbon dioxide is also known as dry ice, but cannot form in the range of temperatures found within Earth's atmosphere, although it is produced industrially for various purposes. So as opposed to water vapour, carbon dioxide is an example of a non-condensing gas: once up there it stays up there for a long time. That's why our vast CO2 emissions are causing levels of the gas to build and build.
If you needed a smoking gun in order to accept the above, there is one: carbon dioxide derived from fossil fuel burning has a very distinct chemical fingerprint that is readily measurable in air samples. To find out how we do that, read the further details below. Such measurements, with a trend precisely following the pathway we would expect due to our increasing emissions, show that far from being someone else's problem, one way or another we are all bang to rights in this case.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
If you wanted to find a smoking gun with respect to human carbon emissions and their role in global warming, where would you look? There are several and the Intermediate version of the rebuttal lists and describes many of them, but we’ll start here with the most important one.
Fossil fuel production and usage is all well-documented. For that reason, CO2 emissions are also well-documented. In 2019, some 44.25 billion tons (or gigatons) were emitted to the atmosphere (IPCC AR6, WG III Technical Summary 2022). That figure was the highest recorded in a steady year-on year upward annual trend.
So we know all too well that every year of late we have added tens of billions of tonnes of CO2 to the atmosphere. How do we detect that fossil fuel signature in the air?
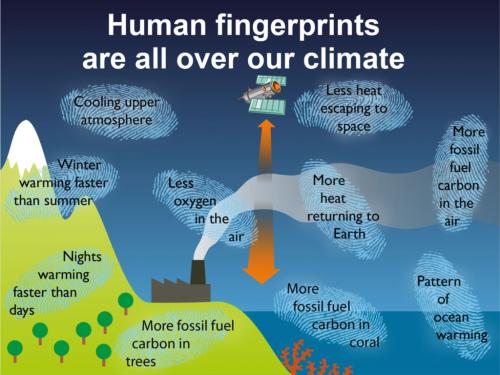
Fig. 1: human fingerprints on the crime-scene of climate change. Note several lines of evidence refer to 'fossil fuel carbon'. Now read on….
The answer lies in carbon isotopes.
Most chemical elements exist in nature as more than one version. These different versions are an element's isotopes. Carbon is no exception to the rule. Its most important natural isotopes are carbon 12, carbon 13 and carbon 14, written 12C, 13C and 14C. All three contain six protons in their atomic nuclei – that's carbon's atomic number and it's fixed. But carbon 12 atomic nuclei contain six neutrons whereas 13C has seven and 14C has eight.
Carbon 14 only occurs in tiny traces – about one atom per gram of carbon is a ballpark figure. It forms in nature through neutron bombardment of nitrogen atoms from cosmic rays near the top of our atmosphere. Man has had a hand here too: nuclear explosions also produce high-energy neutrons and the weapons-testing mania last century increased the amount of 14C by two orders of magnitude. The isotope is useful since it is radioactive and can be used to radiometrically date geologically young materials. Because its decay-rate is rapid (half-life of 5,700 years), anything more than about 50,000 years old is too 14C-depleted for radiocarbon dating. The fossil fuels, millions to hundreds of millions of years old, are devoid of 14C.
Carbon 12 is stable and very common, constituting 98.93% of all carbon on Earth, with carbon 13 making up the remainder, apart from the tiny amount of carbon 14. In that vital process of photosynthesis, carbon dioxide and water are absorbed by plants. These raw ingredients are converted into nutrients (sugar) and as a by-product, two thirds of their contained oxygen are released to the atmosphere, making it breathable by us and our fellow life-forms. The important bit is that during photosynthesis, carbon isotopes fractionate, meaning that the proportions of 12C and 13C are changed by the chemical reactions involved, with a preferential uptake of 12C in that sugar. So photosynthesis produces a shift in favour of 12C.
Any carbon-bearing sample – a bottle of oil, lump of coal, a piece of calcium carbonate such as limestone or a sea-shell, a flask of air - there are numerous examples – can be analysed and its carbon isotopic composition determined. Therefore, its ratio of 13C to 12C (known as delta or d13C) can be calculated and compared to an internationally-agreed standard composition. The equation is as follows:
delta 13C = ((13C/12C sample)/(13C/12C standard) -1) x 1000%
Now, because of that preferential take-up of 12C in plants, the d13C value, expressed in ‰, of anything derived from their decomposition, combustion, preservation or consumption will be similar. As such the smaller (or more negative) d13C value spreads up the food chain, gets preserved in coal, oil or gas deposits, in carbonate rocks like limestones and in shelly fossils.
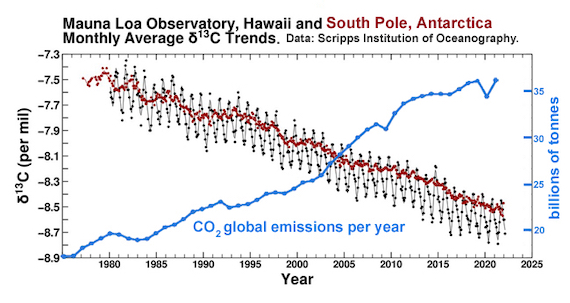
It should come as no surprise, then, that if you dig up and set fire to fossil fuels, the CO2 emitted in that process will give the atmosphere a more negative d13C – and that's exactly what we find. If all the CO2 in the atmosphere was that outgassed by volcanoes, we would see d13C closer to zero; instead the ongoing trend is more and more negative; a cumulative plot from the Mauna Loa and South Pole air sampling stations (fig. 1) shows values of -7.5‰ in 1980, heading steadily downwards to -8.5‰ in 2020. The estimated pre-industrial value is around -6.6‰ (Graven et al. 2020). This is one smoking gun of man-made carbon dioxide emissions. Another is that because the fossil fuels are devoid of 14C, it follows that fossil fuel CO2 emissions are, too - thereby further diluting the already tiny amount of 14C in the atmosphere.
Fig. 1: the carbon isotope record, 1975-2022. Black Dots: Monthly average carbon isotope ratio (d13C) of atmospheric carbon dioxide at Mauna Loa Observatory, Hawai. As with the Keeling Curve of CO2 levels, the graph shows the seasonal wobble caused by photosynthetic plants as leaves grow and then die.Red Dots: Monthly average d13C of atmospheric carbon dioxide at the South Pole, Antarctica.
Carbon isotope ratios are also very useful in geology. Sudden changes in their values, known as positive or negative “excursions”, tell us something monumental has occurred. For example, one of the biggest negative d13C excursions in the geological record marks the end-Permian mass extinction, 250 million years ago (Saitoh & Isozaki 2021).
That one of the most prolonged and voluminous episodes of volcanism in the past 500 million years occurred at the same time as the end Permian mass extinction suggests a lot of CO2 was released back at the time. But volcanogenic CO2, originating in Earth's Mantle, has a “heavier” or more positive d13C of around -6‰. It cannot have caused the negative excursion, but one thing could. In the sedimentary basin through which the magma rose from deep in the Earth there were vast oil and coal deposits and they got comprehensively roasted in the process. It is estimated that as a consequence, the Siberian Traps eruptions released between ten trillion and one hundred trillion tons of carbon dioxide over just a few tens of thousands of years, some of volcanic origin but a heck of a lot from those cooked fossil fuel deposits. What happened in Siberia 250 million years ago thereby presents Mankind with its starkest possible warning about messing with Earth's carbon cycle.
Last updated on 2 July 2023 by John Mason. View Archives































 Arguments
Arguments






































I think I prefer this way - otherwise "It's not happening" and "It's not us" will be #1 and #2 which is a bit too general for my liking.