OA not OK part 1
Posted on 1 July 2011 by Doug Mackie
This post is number 1 in a series about ocean acidification. Other posts: Introduction , 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12,13, 14, 15, 16, 17, 18,Summary 1 of 2, Summary 2 of 2.
Ocean acidification, OA, is very much the other CO2 problem. Ocean acidification is the process of ocean pH decreasing (i.e. becoming more acidic) due to absorption of fossil fuel CO2 from the atmosphere. Another effect of ocean acidification is to reduce the amount of carbonate that is available to marine organisms, such as shellfish, for making their calcium carbonate shells.
The purpose of this post, the first in a twice-weekly series of posts about Ocean Acidification, is to introduce a single chemical equation. We don't want to lapse into hyperbole but the equation is the E = mc2 of ocean acidification. Having said that, knowing E=mc2 does not confer knowledge about element formation during a supernova. Likewise, this equation will only be the start of our learning:
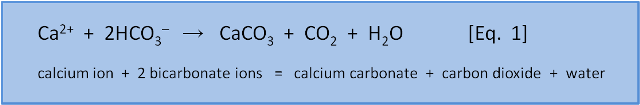
We need a few words about chemical equations before we discuss what Equation 1 tells us. On the left side of the arrow is the calcium ion Ca2+ (an ion is an atom or molecule that has an electrical charge, in this case 2+ meaning calcium has lost 2 negatively charged electrons to establish a +2 charge). Also on the left are 2 bicarbonate ions (HCO3-, with the negative charge meaning it has gained an extra electron). The arrow indicates that the ions on the left of the arrow react to give the molecules on the right of the arrow: calcium carbonate (CaCO3), carbon dioxide (CO2), and water (H2O).
This equation describes the formation of calcium carbonate (i.e. shells) from calcium ions and bicarbonate ions. It shows that making 1 molecule of CaCO3 from a calcium ion requires 2 molecules of bicarbonate (HCO3–) and releases 1 molecule of H2O and 1 molecule of CO2. Yes, you read that right: The formation of calcium carbonate shells is a source of CO2, not a sink for CO2.
![]()
A basic principle is that chemical equations must be balanced. That is, they have the same number and types of atoms on both sides. Counting up we see on both the left and the right are 1 calcium (Ca), 2 hydrogen (H), 2 carbon (C), and 6 oxygen (O) atoms.
However, not all balanced chemical equations are valid chemical equations. The trick of chemistry (Oh! there's that word again) is in knowing if a particular balanced equation is valid. For example, a simple balanced equation can be written for the melting of ice at room temperature and sea level pressure:

But we can also write a balanced equation for the reverse reaction:

The problem is that, despite being able to write a balanced equation, liquid water does not spontaneously freeze at room temperature, so the second equation must be wrong. Actually what is wrong is the direction of the arrow. Reverse the arrow and Equation 2 becomes Equation 3.
Next post: the water example is familiar and we know the right answer from personal experience. But can we predict outcomes without doing the experiment? Yes – if we use thermodynamics.
Written by Doug Mackie, Christina McGraw , and Keith Hunter . This post is number 1 in a series about ocean acidification. Other posts: Introduction , 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.































 Arguments
Arguments
































[DB] You were doing it right, but the WYSIWYG editor conjugates it.
<sup> superscript </sup>
<sub> subscript </sub>
[DB] Your concern is noted, but misplaced and premature. Let us sit back and let the experts first share their knowledge before we students critique it.
That would be the scientific thing to do.
Could someone check out this interactive on sea chemisty and tell me if its incorrect?
www.whoi.edu/oceanus/feature/a-quest-for-resilient-reefs
The interactive on the right, thank you.