The greenhouse effect and the 2nd law of thermodynamics
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
| |||
|
The atmosphere of the Earth is less able to absorb shortwave radiation from the Sun than thermal radiation coming from the surface. The effect of this disparity is that thermal radiation escaping to space comes mostly from the cold upper atmosphere, while the surface is maintained at a substantially warmer temperature. This is called the "atmospheric greenhouse effect", and without it the Earth's surface would be much colder. |
|||||
2nd law of thermodynamics contradicts greenhouse theory
"The atmospheric greenhouse effect, an idea that many authors trace back to the traditional works of Fourier 1824, Tyndall 1861, and Arrhenius 1896, and which is still supported in global climatology, essentially describes a fictitious mechanism, in which a planetary atmosphere acts as a heat pump driven by an environment that is radiatively interacting with but radiatively equilibrated to the atmospheric system. According to the second law of thermodynamics such a planetary machine can never exist." (Gerhard Gerlich)
Most participants in climate debates can agree that the atmosphere's capacity to interact with thermal radiation helps maintain the Earth's surface temperature at a livable level. The Earth's surface is about 33 degrees Celsius warmer than required to radiate back all the absorbed energy from the Sun. This is possible only because most of this radiation is absorbed in the atmosphere, and what actually escapes out into space is mostly emitted from colder atmosphere.
This absorption is due to trace gases which make up only a very small part of the atmosphere. Such gases are opaque to thermal radiation, and are called "greenhouse gases". The most important greenhouse gases on Earth are water vapor and carbon dioxide, with additional contributions from methane, nitrous oxide, ozone, and others. If the atmosphere was simply a dry mix of its major constituents, Oxygen and Nitrogen, the Earth would freeze over completely.
Observing the greenhouse effect in action
The simplest direct observation of the greenhouse effect at work is atmospheric backradiation. Any substance that absorbs thermal radiation will also emit thermal radiation; this is a consequence of Kirchoff's law. The atmosphere absorbs thermal radiation because of the trace greenhouse gases, and also emits thermal radiation, in all directions. This thermal emission can be measured from the surface and also from space. The surface of the Earth actually receives in total more radiation from the atmosphere than it does from the Sun.
The net flow of radiant heat is still upwards from the surface to the atmosphere, because the upwards thermal emission is greater than the downwards atmospheric backradiation. This is a simple consequence of the second law of thermodynamics. The magnitude of the net flow of heat is the difference between the radiant energy flowing in each direction. Because of the backradiation, the surface temperature and the upwards thermal radiation is much larger than if there was no greenhouse effect.
Atmospheric backradiation has been directly measured for over fifty years. The effects of greenhouse gases stand out clearly in modern measurements, which are able to show a complete spectrum.
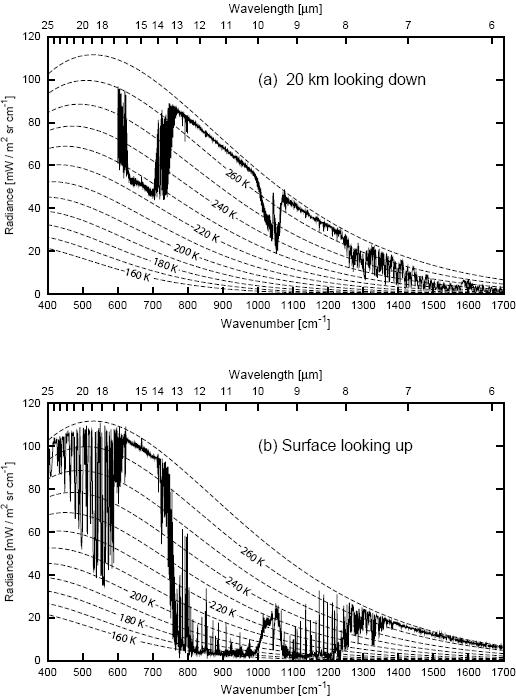
Figure 1. Coincident measurements of the infrared emission spectrum of the cloudfree atmosphere at (a) 20km looking downward over the Arctic ice sheet and (b) at the surface looking upwards. (Data courtesy of David Tobin, Space Science and Engineering Center, University of Wisconsin-Madison. Diagram courtesy of Grant Petty, from Petty 2006).
When you look down from aircraft at 20km altitude (Fig 1a), what is "seen" is the thermal radiation from Earth that gets out to that height. Some of that radiation comes from the surface. This is the parts of the spectrum that follow a line corresponding in the diagram to about 268K. Some of that radiation comes from high in the atmosphere, where it is much colder. This is the parts of the spectrum that follow a line of something like 225K. The bites taken out of the spectrum are in those bands where greenhouse gases absorb radiation from the surface, and so the radiation that eventually escapes to space is actually emitted high in the atmosphere.
When you look up from the surface (Fig 1b), what is "seen" is thermal backradiation from the atmosphere. In some frequencies, thermal radiation is blocked very efficiently, and the backradiation shows the temperature of the warm air right near the surface. In the "infrared window" of the atmosphere, the atmosphere is transparent. In these frequencies, no radiation is absorbed, no radiation is emitted, and here is where IR telescopes and microwave sounding satellites can look out to space, and down to the surface, respectively.
The smooth dotted lines in the diagram labeled with temperatures are the curves for a simple blackbody radiating at that temperature. Water vapor has complex absorption spectrum, and it is not well mixed in the atmosphere. The emissions seen below 600 cm-1 are due to water vapor appearing at various altitudes. Carbon dioxide is the major contributor for emission seen between between about 600 and 750 cm-1. The patch of emission just above 1000 cm-1 is due to ozone.
The term "greenhouse"
The term "greenhouse" was coined for this atmospheric effect in the nineteenth century. A glass greenhouse and an atmospheric greenhouse both involve a physical barrier that blocks the flow of heat, leading to a warmer temperature below the barrier. The underlying physics is different, however. A glass greenhouse works primarily by blocking convection, and an atmospheric greenhouse works primarily by blocking thermal radiation, and so the comparison is not exact. This difference is well understood and explained in most introductions to the subject. Where confusion arises, it is usually the glasshouse that is improperly described, rather than the atmospheric greenhouse effect.
The enhanced greenhouse effect
The greenhouse effect itself has always been an important effect on Earth's climate, and it is essential for maintaining a livable environment. Without it, the surface would rapidly freeze.
The existence of a greenhouse effect itself should not be confused with changes to the greenhouse effect. Global warming in the modern era is being driven by increasing concentrations of greenhouse gases in the atmosphere, which leads to an enhanced greenhouse effect. This is covered in more detail as a separate argument: How do we know more CO2 is causing warming?
Intermediate rebuttal written by John Cook
Update June 2023:
For additional links to relevant blog posts, please look at the "Further Reading" box, below.
Last updated on 29 June 2023 by John Mason. View Archives































 Arguments
Arguments



































Climate Myth...