Is the CO2 effect saturated?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
The notion that the CO2 effect is 'saturated' is based on a misunderstanding of how the greenhouse effect works. |
|||||||
Climate Myth...
CO2 effect is saturated
"Each unit of CO2 you put into the atmosphere has less and less of a warming impact. Once the atmosphere reaches a saturation point, additional input of CO2 will not really have any major impact. It's like putting insulation in your attic. They give a recommended amount and after that you can stack the insulation up to the roof and it's going to have no impact." (Marc Morano, as quoted by Steve Eliot)
At-a-Glance
This myth relies on the use (or in fact misuse) of a particular word – 'saturated'. When someone comes in from a prolonged downpour, they may well exclaim that they are saturated. They cannot imagine being any wetter. That's casual usage, though.
In science, 'saturated' is a strictly-defined term. For example, in a saturated salt solution, no more salt will dissolve, period. But what's that got to do with heat transfer in Earth's atmosphere? Let's take a look.
Heat-trapping by CO2 in the atmosphere happens because it has the ability to absorb and pass on infra-red radiation – it is a 'greenhouse gas'. Infra-red is just one part of the electromagnetic spectrum, divided by physicists into a series of bands. From the low-frequency end of the spectrum upwards, the bands are as follows: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. Gamma rays thus have a very high-frequency. They are the highest-energy form of radiation.
As our understanding of the electromagnetic spectrum developed, it was realised that the radiation consists of particles called 'photons', travelling in waves. The term was coined in 1926 by the celebrated physicist Gilbert Lewis (1875-1946). A photon's energy is related to its wavelength. The shorter the wavelength, the higher the energy, so that the very high-energy gamma-rays have the shortest wavelength of the lot.
Sunshine consists mostly of ultraviolet, visible light and infra-red photons. Objects warmed by the sun then re-emit energy photons at infra-red wavelengths. Like other greenhouse gases, CO2 has the ability to absorb infra-red photons. But CO2 is unlike a mop, which has to be wrung out regularly in order for it to continue working. CO2 molecules do not get filled up with infra-red photons. Not only do they emit their own infra-red photons, but also they are constantly colliding with neighbouring molecules in the air. The constant collisions are important. Every time they happen, energy is shared out between the colliding molecules.
Through those emissions and collisions, CO2 molecules constantly warm their surroundings. This goes on all the time and at all levels in the atmosphere. You cannot say, “CO2 is saturated because the surface-emitted IR is rapidly absorbed”, because you need to take into account the whole atmosphere and its constant, ongoing energy-exchange processes. That means taking into account all absorption, all re-emission, all collisions, all heating and cooling and all eventual loss to space, at all levels.
If the amount of radiation lost to space is equal to the amount coming in from the Sun, Earth is said to be in energy balance. But if the strength of the greenhouse effect is increased, the amount of energy escaping falls behind the amount that is incoming. Earth is then said to be in an energy imbalance and the climate heats up. Double the CO2 concentration and you get a few degrees of warming: double it again and you get a few more and on and on it goes. There is no room for complacency here. By the time just one doubling has occurred, the planet would already be unrecognisable. The insulation analogy in the myth is misleading because it over-simplifies what happens in the atmosphere.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
This myth relies on the use of a word – saturated. When we think of saturated in everyday use, the term 'soggy' comes to mind. This is a good example of a word that has one meaning in common parlance but another very specific one when thinking about atmospheric physics. Other such words come to mind too. Absorb and emit are two good examples relevant to this topic and we’ll discuss how they relate to atmospheric processes below.
First things first. The effect of CO2 in the atmosphere is due to its influence on the transport of 'electromagnetic radiation' (EMR). EMR is energy that is moving as x-rays, ultraviolet (UV) light, visible light, infrared (IR) radiation and so on (fig. 1). Radiation is unusual in the sense that it contains energy but it is also always moving, at the speed of light, so it is also a form of transport. Radiation is also unusual in that it has properties of particles but also travels with the properties of waves, so we talk about its wavelength.
The particles making up radiation are known as photons. Each photon contains a specific amount of energy, and that is related to its wavelength. High energy photons have short wavelengths, and low energy photons have longer wavelengths. In climate, we are interested in two main radiation categories - firstly the visible light plus UV and minor IR that together make up sunshine, and secondly the IR from the earth-atmosphere system.
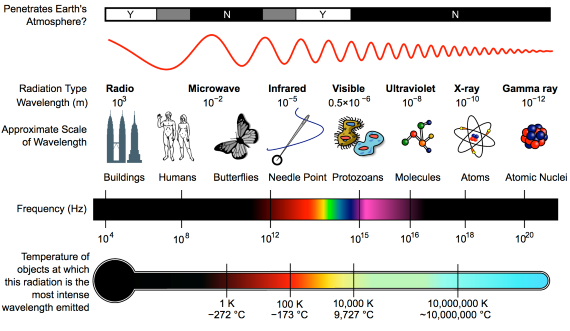
Fig. 1: diagram showing the full electromagnetic spectrum and its properties of the different bands. Image: CC BY-SA 3.0 from Wikimedia.
CO2 has the ability to absorb IR photons – it is a 'greenhouse gas'.So what does “absorb” mean, when talking about radiation? We are all familiar with using a sponge to mop up a water spill. The sponge will only absorb so much and will not absorb any more unless it's wrung out. In everyday language it may be described, without measurements, as 'saturated'. In this household example, 'absorb' basically means 'soak up' and 'saturated' simply means 'full to capacity'. Scientific terms are, in contrast, strictly defined.
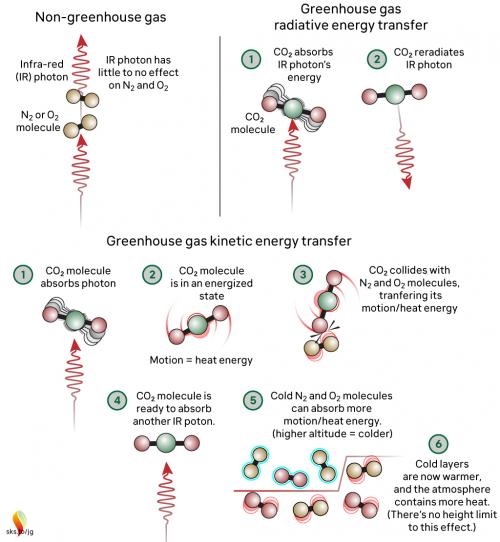
Now let's look at the atmosphere. The greenhouse effect works like this: energy arrives from the sun in the form of visible light and ultraviolet radiation. A proportion reaches and warms Earth's surface. Earth then emits the energy in the form of photons of IR radiation.
Greenhouse gases in the atmosphere, such as CO2 molecules, absorb some of this IR radiation, then re-emit it in all directions - including back to Earth's surface. The CO2 molecule does not fill up with IR photons, running out of space for any more. Instead, the CO2 molecule absorbs the energy from the IR photon and the photon ceases to be. The CO2 molecule now contains more energy, but that is transient since the molecule emits its own IR photons. Not only that: it's constantly colliding with other molecules such as N2 and O2 in the surrounding air. In those collisions, that excess energy is shared with them. This energy-sharing causes the nearby air to heat up (fig. 2).
Fig. 2: The greenhouse effect in action, showing the interactions between molecules. The interactions happen at all levels of the atmosphere and are constantly ongoing. Graphic: jg.
The capacity for CO2 to absorb photons is almost limitless. The CO2 molecule can also receive energy from collisions with other molecules, and it can lose energy by emitting IR radiation. When a photon is emitted, we’re not bringing a photon out of storage - we are bringing energy out of storage and turning it into a photon, travelling away at the speed of light. So CO2 is constantly absorbing IR radiation, constantly emitting IR radiation and constantly sharing energy with the surrounding air molecules. To understand the role of CO2, we need to consider all these forms of energy storage and transport.
So, where does 'saturation' get used in climate change contrarianism? The most common way they try to frame things is to claim that IR emitted from the surface, in the wavelengths where CO2 absorbs, is all absorbed fairly close to the surface. Therefore, the story continues, adding more CO2 can’t make any more difference. This is inaccurate through omission, because either innocently or deliberately, it ignores the rest of the picture, where energy is constantly being exchanged with other molecules by collisions and CO2 is constantly emitting IR radiation. This means that there is always IR radiation being emitted upwards by CO2 at all levels in the atmosphere. It might not have originated from the surface, but IR radiation is still present in the wavelengths that CO2 absorbs and emits. When emitted in the upper atmosphere, it can and will be lost to space.
When you include all the energy transfers related to the CO2 absorption of IR radiation – the transfer to other molecules, the emission, and both the upward and downward energy fluxes at all altitudes - then we find that adding CO2 to our current atmosphere acts to inhibit the transfer of radiative energy throughout that atmosphere and, ultimately, into space. This will lead to additional warming until the amount of energy being lost to space matches what is being received. This is precisely what is happening.
The myth reproduced at the top – incorrectly stating an analogy with roof insulation in that each unit has less of an effect - is misleading. Doubling CO2 from 280 ppm to 560 ppm will cause a few degrees of warming. Doubling again (560 to 1130 ppm) will cause a similar amount of additional warming, and so on. Many doublings later there may be a point where adding more CO2 has little effect, but recent work has cast serious doubt on that (He et al. 2023). But we are a long, long way from reaching that point and in any case we do not want to go anywhere near it! One doubling will be serious enough.
Finally, directly observing the specific, global radiative forcing caused by well-mixed greenhouse gases has - to date - proven elusive. This is because of irregular, uncalibrated or limited areal measurements. But very recently, results have been published regarding the deep reinterrogation of years of data (2003-2021) from the Atmospheric Infrared Sounder (AIRS) instrument on NASA's Aqua Satellite (Raghuraman et al. 2023). The work may well have finally cracked the long-standing issue of how to make finely detailed, consistent wavelength-specific measurements of outgoing long-wave radiation from Earth into space. As such, it has opened the way to direct monitoring of the radiative impact (i.e. forcing + feedback) of greenhouse gas concentration changes, thereby complimenting the Keeling Curve - the longstanding dataset of measured CO2 concentrations, down at the planet's surface.
Note: Several people in addition to John Mason were involved with updating this basic level rebuttal, namely Bob Loblaw, Ken Rice and John Garrett (jg).
Last updated on 31 December 2023 by John Mason. View Archives































 Arguments
Arguments





































Hope this is the right place to point out that Figs 1 & 2 have disappeared from the 'Advanced' article, apparently after Altervista suspended their hosting. They're still available in the PDF and via the Wayback Machine.
[DB] Updated. Thanks for the heads-up!
I fail to find convincing evidence as to how CO2 can be the cause of global warming with a occurence of only 400 ppm. This would entail that 1 CO2 molecule would need to heat up 2,500 other molecules in the atmosphere to cause any increase in overall temperature. How is this possibly explainable! It is impossible.
Barcino:
You are making an argument from incredulity. An equivalent counterargument would be for me to say "I can't believe you don't understand how this works". Very easy to say, but carries no weight.
The place you want to look is on the "CO2 is a trace gas" page:
https://skepticalscience.com/CO2-trace-gas.htm
Please read it before you comment again, and place comments on that thread, not this one.
[PS] I am reasonably sure that nothing said by anyone will convince a person that doesnt want to be convinced but lets see. Barcino's statement suggests he hasnt actually read a proper explanation of how the greenhouse effect really works.
@Barcino2017
Are you trolling? Or can you genuinely not come up with an idea of how molecules of CO2 can heat up other molecules in the atmosphere? This idea around 2500 molecules seems to be quite common in denier circles and reminds me of a "discussion" I had with someone who was pushing this idea:
Denier: There are 2500 molecules for each molecule of CO2. For an increase of 1C the CO2 molecule would have to be heated to 2500C.
Me: Why would the CO2 molecule have to be heated to 2500C?
Denier: Because er, 2500, you dishonest green rent grant seeker.
Me: What? Why?
Denier: Your smears and lack of empirical data and science are obvious. You are a dishonest liar. I win!!!!!!!!!!?
Now, you don't want to be that person, do you? Your idea is slightly less extreme than his as you do not propose a CO2 molecule has to be heated all in one flash and heat 2500 other molecules at the same time. But you are suggesting a CO2 molecule cannot heat more than one molecule. Ever. Like, once it has heated another molecule it can never do it again? A one-off never to be repeated event?
Perhaps you need to rethink that misconception.
The facts are obscured by the above analysis. The facts are that the effects of CO2 on the atmospheric absorption of IR by the atmosphere diminish rapidly and logartithmically with increasing concentration, so most of the so called greenhouse effect, that absorbs and re radiates IR, which isn't how greenhouses work, occurs in concentrations below 150ppm, which is so low trees and other vegetation die. Lower than the lowest and ice age minimums.
The effect is approximately the same for each doubling, so has expoentially LESS effect per ppm, so that at 200ppm another 200ppm, to get to where we are now, only produces the same effect as 20-40ppm.
So the statement that its never satuarted is deceitfully true, because it is partial by omitting the dominant factor, that while it's never quire saturated, it may as well be for all the effect it has, versus the natural variation in the 300 times greater effect of Water vapour, for example.
Reference? There is a useful course on this by the University of Chicago you can take for free w/o a certificate - that costs - which gives this initial fact in itshttps://skepticalscience.com/CO2-is-not-the-only-driver-of-climate.htm promo video. These are the facts on IR absorption of CO2 in the atmosphere. Not as advertised. The reverse of a tipping point in fact. We need to be looking elsewhere than CO2 for the rue cause of the tiny variation we are currently observing with the interglacial peak tempertaure range of an ice age interglacial. Soon be over, but at a rate humans cannot detect in a lifetime, as a quick study of the data shows anyone numerate. Real planetary climate hange of an interglacial is significant, but again any effect takes seberal lifetimes to be on bservable, even at the geologically rapid end of the current ice ages, over only 1,000 years...................
https://www.coursera.org/learn/global-warming/lecture/CnAIV/the-band-saturation-effect
[JH] url link activated. Please learn how to do this using the edit function provided.
For future reference, you may want to spell-check your draft text prior to posting a comment.
[TD] SkepticalScience is organized into a large number of narrow topics. You have commented on a topic that is only slightly relevant to your point about the direct IR effect of CO2 increase being logarithmic. Please click the View All Arguments link at the bottom of the list of Most Used Climate Myths, in the left margin, for more relevant posts, such as How Do We Know More CO2 Is Causing Warming?, and CO2 Is Main Driver of Climate Change. Note that many posts have Basic, Intermediate, and Advanced tabbed panes. The log relationship of IR absorption to CO2 level has been known for over 150 years, and always has been explicitly accounted for in all calculations of temperature effects of increased CO2.
[TD] To find posts specifically addressing the logarithmic relationship, enter the word logarithmic in the Search field at the top right of the page.
Brian Catt @455 , from what I've seen of the Uni. Chicago lecture, it does not support the thrust of the statements you have made. Please explain better, what you are intending to say — since your comments are coming across as "confused about the science".
Then there are the simple errors in your statements, such as (A) the current "tiny variation" [unquote] in surface temperature. [Alas, not so. The temperature is shooting upwards like a rocket. There's been nothing like this in the 200,000+ years of human history. And scientifically, the cause is obvious and undisputed. ]
.... And (B) water vapour causing "300 time greater effect" [unquote] in warming compared with CO2. Is that what you think? Or did you mean to state 3 times greater? Because that is around the upper limit of the multiplying "feedback" response by H2O to the driving force of CO2.
BC embarrassed himself, and forever established himself as a denier, on this NOAA thread on Facebook.
Put your coffee down before reading.
Daniel, "This page isn't available
The link you followed may be broken, or the page may have been removed."
As a brief background to this comment, I had an encounter with a denialist last week who stated that Arrhenius' observations regarding CO2 as a greenhouse gas had been debunked. In hindsight, I should have asked precisely what he meant by that. Instead, I retorted that this was news to me, at which he remarked that my kind was unlikely to look up the facts (I have a masters degree in Physics, and found this an interesting example of projection rhetoric...). Anyway, here I am.
I'm trying to follow the argument here, so that I can express it in layman's terms. It seems the idea of greenhouse warming isn't as straightforward as I thought. I get Angstrom's counter-argument to Arrhenius about current CO2 levels absorbing all IR radiation long before it has a chance to escape the atmosphere and that, therefore, adding more CO2 won't alter things. I'm having a problem grasping the explanation in response the Angstrom being put forward here. If I may paraphrase what I understand of it:
1. IR photon from surface is repeatedly absorbed and re-emitted (by CO2) in random directions (losing a little energy each time). Check.
2. Photon is finally able to escape into space at a high enough altitude. Check.
3. Adding more CO2 increases the density, and hence the altitude this escape occurs. Check.
4. Being higher means lower temperature, and hence less energy is emitted. Errm... as a general consequence of a blackbody spectrum, sure. As a discrete energy photon emission, of what significance is the temperature?
I'm not saying the explanation's wrong, but it is very hard to follow, and is even counterintuitive (we're talking about rising temperatures, and then lowered temperatures? It's hard enough for me to follow, let alone someone without a scientific background)
My own quick explanation would be that increasing CO2 concentrations reduces the mean free path of IR photons, and that increases the number of scattering (heating) events before the photons can escape. It seems a lot more concise and intuitive than the explanation put here, but is it right? What am I missing?
Arf @459 , the usual explanations follow the course of IR photons (of the bandwidth absorbed/emitted by CO2 molecules) as they are radiated out from the warm surface of the land/ocean. Layer after layer of atmosphere absorbs and re-emits IR photons in all directions (as you are already aware, of course).
Rising through air, as the layers become less dense, the individual photon "journeys" (between CO2 atoms) become longer — yet each same-depth layer is still emitting the same previous total of upwards and downwards amount of IR radiation (of course). Only in the most tenuous uppermost layers, does this "stacking" of upward/downward emissions begin to break down, as an increasing percentage of upward IR photons evade reabsorption and make a straightline escape to outer space. In effect, we can think of the upper atmosphere as producing only back-radiation (at this particular bandwidth we are interested in) as far as the Earth is concerned.
I am sure I am telling you nothing new, in all this. I will point out that at an individual level, each IR photon maintains its same energy level, as it is "reincarnated" — the individual CO2 molecule recipient of photonic energy "cools itself" by imparting kinetic energy to a neighbouring N2 or O2 . . . and at a later time regains energy kinetically from a neighbouring N2 molecule, and "reincarnates" & emits an IR photon of the same energy level as previously received but in a random direction. Of course, as intermolecular distances increase, and the kinetic temperature of N2 molecules reduces, then these "deaths & reincarnations" of IR photons (per second per cubic mm) must reduce. But the final total product is back-radiation towards the Earth's surface plus upwards "lost" radiation (and of course the totality of all "lost" radiation over the whole spectrum must equal what's originally entered the planetary system from the sun, at equilibrium — or at least extremely close to that total while the system is in transition to equilibrium).
Myself, I find it easier to mentally picture these events if you rotate the Earth surface 90 degrees. Instead of a horizontal surface emitting upwards, choose to picture the surface as the y-axis and the atmosphere layers stacked outwards along the x-axis. The cool outermost layers of air are losing radiation outwards to space, and are emitting "back-radiation" inwards. Through the bulk of the atmosphere, each layer is transmitting fractionally more energy inwards than outwards, and these fractional differences integrate to produce a gradient of temperature, highest at the surface and "sloping down" to the outermost air. Hence the surface is warmer than the outermost air.
When the atmospheric CO2 concentration becomes raised, the x-axis is extended further (so to speak) . . . and the same gradient produces a higher cumulative back-radiation at the planetary surface: in other words, the surface becomes warmer than under the previous conditions.
Arf,
My understanding is a little different from yours, just a little. To review your points:
1) I think the energy of photons is fixed so energy is not lost each time. I am not sure what you mean by lost energy. This point is not important.
2) Check.
3) Check.
4) This is the important step for the greenhouse effect. We agree that the escape altitude increases.
As you know the amount of energy radiated is related to the temperature. The temperature at the escape altitude must be high enough for all the energy that comes from the sun to escape to conserve energy. This fixes the temperature at the escape altitude. When more greenhouse gas is added the temperature at the new altitude increases so that energy is conserved.
The temperature in the troposphere (the lower atmosphere) increases as you decrease in altitude. The rate of increase is called the lapse rate. The lapse rate is about 6.5C per kilometer. The lapse rate is a physical property of the atmosphere and is fixed by basic physics and chemistry laws and properties.
The temperature at the altitude of escape is fixed by the law of conservation of energy and the temperature varies in the atmosphere according to the lapse rate. When the altitude of escape is increased that results in an increase in temperature at that altitude. This is then passed down to the surface according to the lapse rate.
It is interesting to note that an increase in the altitude of only 100 meters results in an increase in the surface temperature of 0.65C. 2C is only 300 meters increase in escape altitude.
On Venus the CO2 concentration is much higher so the altitude of energy escape is much higher. That results is the surface of Venus being about 462C. This temperature can be calculated using the equations that describe the greenhouse effect on Earth.
Your explaination seems OK to me but does not well describe the changes in the atmosphere.
Hi all, i'd like to follow on from Arf @459 above. It doesn't seem his query on why the temperature of the upper atmosphere matters when we're talking about discrete emission of a photon has been adequately addressed. The comment at 461 seems contradictory, as if there is an increase in temperature at the altitude of escape then this will increase black body radiation to space...
My other query relates to how the co2-absorbed photons actually contribute to warming, if the re-emitted photons are of the same energy as the absorbed ones. Is it that through a kinetic effect where a proportion of high energy absorbed co2 molecules vibrate surrounding air molecules, losing the absorbed energy and therefore never re-emitting the photon? Or is it because a proportion of the re-emitted photons are absorbed by something else (water, the ground etc). Or both?
I assume the photons we're talking about make their way out pf the atmosphere through a random walk type process
LTO: Contrary to your second sentence, the temperature at the altitude of escape decreases, not increases. That is not because the temperature at a given altitude (of the troposphere) decreases. Instead, it is because the altitude of escape increases, and higher altitudes have lower temperatures. Increase in temperature of a given altitude due to greater CO2 absorbing IR from below, is insufficient to counteract the lower temperature due to the higher altitude of escape. The altitude of escape increases because of the larger number of CO2 molecules between a given altitude and outer space.
Regarding your second query: A CO2 molecule collides with other molecules--CO2 or other--about 100,000 times more often than that CO2 molecule emits a photon. So the vast majority of the time, the energy a CO2 molecule acquires from absorbing a photon is transferred to other molecules. That is part of the reason why Mars is so cold despite having so much more CO2 than the Earth does. Mars has so few molecules of anything in its atmosphere, there are few transfers of energy to other molecules, so much more of the energy remains in the CO2 molecule until it is emitted as a photon.
Yes, photons are emitted from CO2 molecules in random directions.
Goodbye man-made global warming? As an independent (i.e. impartial) consulting geologist (doctorate in sedimentary geology) with 35 years of experience, having conducted an unpaid (impartial) full-time 3-year (since Nov 2015; continuing) review of the literature from ALL scientific disciplines relevant to climate- and sea-level change (geology, archaeology, physics, astrophysics, oceanography, meterorology, etc, etc), here are my main conclusions:
(1) There's obviously no doubt that Earth has warmed since thermometer measurements began in the 1800s (HadCRUT data; and online NASA/GISS online charts [yearly, monthly, and others], updated every few weeks). However, Earth began COOLING in February 2016 (NASA/GISS monthly chart). This cooling already exceeds all other measured coolings since 1995, in both duration (nearly 3 years so far) and magnitude (0.5 degrees C, fully one-third of IPCCs dreaded '1.5 degrees C by 2100', but in the wrong direction) ...
(2) Warming was driven by increasing solar-MAGNETIC output (controlling cosmic rays, therefore cloudiness; Svensmark's breathtakingly elegant theory), nothing to do with mankind's CO2 emissions which just happened, by pure (bad) luck, to grow during a solar upswing (rather than downswing), a ghastly coincidence; the reverse was about equally likely, 50:50.
(3) Changes in temperature are lagging about 25 years behind changes in solar-magnetic output, due to ocean thermal inertia (google it), dismissed by IPCC.
(4) Sea level is about to rise about 3 metres (sic), before 2100, driven by the increase in solar-magnetic output (up until its 1996 peak), its effect on sea level delayed a further 20 years (approx.; i.e. total sea-level lag is about 45 years) due to ocean 'conveyor-belt' circulation (also ignored by IPCC) delaying the arrival at Antarctica of 'solar-overwarmed' Atlantic surface water, via downwelling and southward mid-depth flow (AMOC). The floating ice shelves buttressing Antarctic on-land glaciers are NOW disintegrating at an accelerating rate (led by Pine Island, Thwaites and Totten), so catastrophic glacier failure by MISI and/or MICI is likely to begin within a decade, raising sea level by at least 3m within about 50 years. It's unstoppable.
Am I right? We'll know very soon. Regarding solar control of global temperature, the next two years will tell: I predict continued cooling, so keep a close eye on that NASA temperature chart. Regarding sea level, we'll know within 10 years, possibly much sooner: I predict the rate of sea-level rise, currently a trivial 3mm/year, but already increasing exponentially, will be at least ten times higher (3cm/year) by 2030, if not 2025. Watch NASA's online sea-level chart, updated every few months.
See my 20 ResearchGate contributions, mostly one-page items or single figures, fully self explanatory ... https://www.researchgate.net/project/Imminent-metre-scale-non-anthropogenic-sea-level-rise
[DB] "Earth began COOLING in February 2016"
Statistical significance testing shows that, for climate related changes, 17 years (Santer et al) are the bare minimum, with 30 years or more being typically used.
For ANY of the instrumental series, over ANY time span ending in the present:
Ergo, the warming continues, unabated.
Note that commenting here at Skeptical Science adheres to the nature of the OP of the thread upon which you comment. Please follow that rule. Thousands of comment threads exist here upon virtually every topic related to climate change and the denial of it. Use the search function to find the most appropriate thread for your expansion of your knowledge of the science.
Off-topic snipped.
Geologist-for-a-change [of pseudonym? ] @464 ,
Attention !
# You have posted on the wrong date. It is not yet April 1st.
# You have posted in the wrong thread. This thread is for "CO2 Saturated"-related comments.
# You have posted on the wrong website. You should be on WattsUpWithThat ~ the home website for commenters who have the deluded belief that the scientists are all wrong about everything.
# And you appear to have posted 27 years prematurely. If you have (as you say) studied climate science full-time for 3 years, and have not yet disentangled yourself from (almost) every piece of climate crackpottery known to man . . . then it sounds like you have stepped out no more than 10% of your journey from ignorance to knowledge.
Please return in 2046, and let us know how your education succeeded.
Eclectic @465,
I think your response to the trolling is appropriate. I would perhaps add that the fool cannot even provide the numbers in his first point correctly. The date since which peak monthly surface temperature anomalies exceeded later lower anomalies in duration and in temperature difference was 2008 not 1995. This should be no surprise given the 2010 El Nino wasn't as big as the 2016 or 1998 versoins.
[DB] Inflammatory snipped.
LTO @462,
Regarding the altitude issue, I think the explanations you see as being at variance is due to them being part-explanations.
To explain:- if atmospheric CO2 levels increase, the altitude at which CO2 can emit photons dirctly into space increases. This results in the temperature of the space-emitting CO2 being lower and this lower temperature reduces the number of photons emitted and thus the global energy being lost to space.
These CO2-emitted photons are all in a small part of the IR spectrum with ~15 microns wavelength.
As the global energy is now out-of-balance, global temperature will rise, this temperature rise increasing the photons lost to space over all of the IR spectrum. When the energy balance is restored through this warming, the CO2 emission altitude will still be cooler than the emissions altitude prior to the increase in atmospheric CO2 levels.
And I feel your "other query" hasn't been fully addressed.
A photon at the right energy (ie wavelength) can be absorbed by a CO2 molecule and set it into a bendy wobble. In almost all these occurances, the CO2 molecule will then be involved in a collision with another air molecule and the photon's energy will be absorbed within the gas, it being transferred to other modes of gas energy. You ask what then happens to this energy. It will be passed around the gas, this constituting a temperature increase. But a temperature increase will also mean more CO2 molecules are being walloped by the air molecules about them and this will result in more of them being in that bendy wobble which allows them to emit a photon. So more temperature also means more photons emitted by CO2, this cooling the gas, this providing an energy balncing mechanism. And note that if the number of absorbed photons increases because of more CO2, there is also more CO2 to go into a bendy wobble and then to emit photons, which also balances out the energy equation.
Of course, those are still much-simplified descriptions.
MA, Tom: really appreciate your replies, explains some points but also raise a few things I don't quite follow.
1. How significant is the effect of temperature on the likelihood of excited CO2 photon release? At higher altitude the atmospheric pressure is also decreased, which means that the length of time between molecule collisions is also increased. Similarly lower temperatures will decrease the rates of collision. Part of the issue here is the reporting of CO2 in ppm only - given the changes in pressure and temperature I'd have thought you needed accompanying concentration /pressure / temp data to really makes sense of how the competing phenomena interact.
2. Do you know what altitude range the CO2 photon to space release currently happens, how its changed and how sensitive this is to CO2 concentration? As I understand it the temperature actually increases between the tropopause (~11 km) and stratopause (~50 km). It decreases again to the mesopause (~85 km) and then stays pretty constant. The pressure by contrast drops 10 fold every 15 km or so. (see attached)
3. Is it only the altitude of escape atmospheric layer that is the relevant metric here in determining warming, or is it the depth of the envelope from ground to the altitude throughout which warming occurs. I'm trying to understand where exactly in the atmosphere the warming actually happens, and how this is then reflected back at ground level.
Thanks!
Graph of temperature and pressure by atmospheric height here: https://imgur.com/a/juS7yVf
LTO: Sorry, i’m Not knowledgeable enough to answer those questions.
LTO: ScienceOfDoom has excellent explanations of the greenhouse gas effect.
Thanks Tom - that site looks good, but will take some time to work through before can divine anawrrs to those questions. This is clearly very complicated physics - complicated to the extent that I'm wondering how many people really understand it well enough to be completely confident they have taken account of all complexities given the unknowns in the system, as opposed to just taking someone else's word for it. MA may have the answers!
LTO: Oh, so no one should ever believe or act on any science in any field unless one completely understands everything about it? I’m sorry I wasted the time to answer your questions, because I strongly suspect that you never had any genuine interest in the answers.
LTO,
I thought I could chime in since the original question was about my post.
I think you want a lot of detail and Science of DOom and And Then Theres Physics are good sources of detailed information.
For your questions:
1) The number of collisions is so great that the temperature and pressure do not make much difference. (I found an on line calculator once and was amazed at how many collisions there were and how small the temperature and pressure affected the colision rate).
It is probably too difficult to specify the temperature and pressure since it varies so much in different places. The ppm of CO2 is relatively constant through most of the atmosphere.
2) 10 km is commonly used as the escape altitude. This is a simplification for a basic explaination. The actual escape altitude would be different for different wavelengths, different in the tropics and the Arctic, different in deserts than over water and different over storms versus calm weather. Think of how much warmer it is at night when it is cloudy. Only the temperatures in the troposphere matter to the surface temperature. Increasing CO2 causes the stratosphere and the mesosphere to cool at the same time the troposphere warms! source
3) I think the altitude of escape is the key figure but you should check at Science of Doom. Come back here and post if you find out exactly what the explaination is.
Tom: I'm not sure what I did to attract your ire, but I apologize. The point I was trying to express is that the actual theory behind co2-induced global warming is significantly more complicated than I'd thought for a long time. To the extent that I genuinely wonder how many people would really claim to fully understand it, scientists (of which I'm one, albeit not a physicist) included. Is there anyone on this site who would put themselves in that category?
The impression I get (which may not be accurate) from that science of doom website is that the theory is based on modelling of complex interrelated agonistic and antagonostic phenomena, which makes it somewhat different class of scientific theory. Models are necessarily simplifications, and in highly complex systems hidden variables can often lead to very unexpected effects in the real world, which therefore leaves room for reasonable skepticism and uncertainty. Anyway, I'm on a journey of discovery here and have already learnt a lot, in part thanks to you, so once again thank you for your help and I'm sorry you feel you wasted your time.