Is the CO2 effect saturated?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
The notion that the CO2 effect is 'saturated' is based on a misunderstanding of how the greenhouse effect works. |
|||||||
Climate Myth...
CO2 effect is saturated
"Each unit of CO2 you put into the atmosphere has less and less of a warming impact. Once the atmosphere reaches a saturation point, additional input of CO2 will not really have any major impact. It's like putting insulation in your attic. They give a recommended amount and after that you can stack the insulation up to the roof and it's going to have no impact." (Marc Morano, as quoted by Steve Eliot)
At-a-Glance
This myth relies on the use (or in fact misuse) of a particular word – 'saturated'. When someone comes in from a prolonged downpour, they may well exclaim that they are saturated. They cannot imagine being any wetter. That's casual usage, though.
In science, 'saturated' is a strictly-defined term. For example, in a saturated salt solution, no more salt will dissolve, period. But what's that got to do with heat transfer in Earth's atmosphere? Let's take a look.
Heat-trapping by CO2 in the atmosphere happens because it has the ability to absorb and pass on infra-red radiation – it is a 'greenhouse gas'. Infra-red is just one part of the electromagnetic spectrum, divided by physicists into a series of bands. From the low-frequency end of the spectrum upwards, the bands are as follows: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. Gamma rays thus have a very high-frequency. They are the highest-energy form of radiation.
As our understanding of the electromagnetic spectrum developed, it was realised that the radiation consists of particles called 'photons', travelling in waves. The term was coined in 1926 by the celebrated physicist Gilbert Lewis (1875-1946). A photon's energy is related to its wavelength. The shorter the wavelength, the higher the energy, so that the very high-energy gamma-rays have the shortest wavelength of the lot.
Sunshine consists mostly of ultraviolet, visible light and infra-red photons. Objects warmed by the sun then re-emit energy photons at infra-red wavelengths. Like other greenhouse gases, CO2 has the ability to absorb infra-red photons. But CO2 is unlike a mop, which has to be wrung out regularly in order for it to continue working. CO2 molecules do not get filled up with infra-red photons. Not only do they emit their own infra-red photons, but also they are constantly colliding with neighbouring molecules in the air. The constant collisions are important. Every time they happen, energy is shared out between the colliding molecules.
Through those emissions and collisions, CO2 molecules constantly warm their surroundings. This goes on all the time and at all levels in the atmosphere. You cannot say, “CO2 is saturated because the surface-emitted IR is rapidly absorbed”, because you need to take into account the whole atmosphere and its constant, ongoing energy-exchange processes. That means taking into account all absorption, all re-emission, all collisions, all heating and cooling and all eventual loss to space, at all levels.
If the amount of radiation lost to space is equal to the amount coming in from the Sun, Earth is said to be in energy balance. But if the strength of the greenhouse effect is increased, the amount of energy escaping falls behind the amount that is incoming. Earth is then said to be in an energy imbalance and the climate heats up. Double the CO2 concentration and you get a few degrees of warming: double it again and you get a few more and on and on it goes. There is no room for complacency here. By the time just one doubling has occurred, the planet would already be unrecognisable. The insulation analogy in the myth is misleading because it over-simplifies what happens in the atmosphere.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
This myth relies on the use of a word – saturated. When we think of saturated in everyday use, the term 'soggy' comes to mind. This is a good example of a word that has one meaning in common parlance but another very specific one when thinking about atmospheric physics. Other such words come to mind too. Absorb and emit are two good examples relevant to this topic and we’ll discuss how they relate to atmospheric processes below.
First things first. The effect of CO2 in the atmosphere is due to its influence on the transport of 'electromagnetic radiation' (EMR). EMR is energy that is moving as x-rays, ultraviolet (UV) light, visible light, infrared (IR) radiation and so on (fig. 1). Radiation is unusual in the sense that it contains energy but it is also always moving, at the speed of light, so it is also a form of transport. Radiation is also unusual in that it has properties of particles but also travels with the properties of waves, so we talk about its wavelength.
The particles making up radiation are known as photons. Each photon contains a specific amount of energy, and that is related to its wavelength. High energy photons have short wavelengths, and low energy photons have longer wavelengths. In climate, we are interested in two main radiation categories - firstly the visible light plus UV and minor IR that together make up sunshine, and secondly the IR from the earth-atmosphere system.
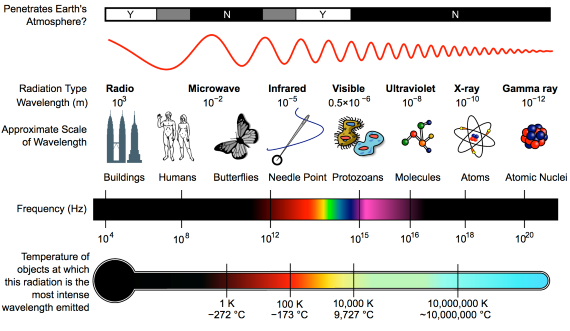
Fig. 1: diagram showing the full electromagnetic spectrum and its properties of the different bands. Image: CC BY-SA 3.0 from Wikimedia.
CO2 has the ability to absorb IR photons – it is a 'greenhouse gas'.So what does “absorb” mean, when talking about radiation? We are all familiar with using a sponge to mop up a water spill. The sponge will only absorb so much and will not absorb any more unless it's wrung out. In everyday language it may be described, without measurements, as 'saturated'. In this household example, 'absorb' basically means 'soak up' and 'saturated' simply means 'full to capacity'. Scientific terms are, in contrast, strictly defined.
Now let's look at the atmosphere. The greenhouse effect works like this: energy arrives from the sun in the form of visible light and ultraviolet radiation. A proportion reaches and warms Earth's surface. Earth then emits the energy in the form of photons of IR radiation.
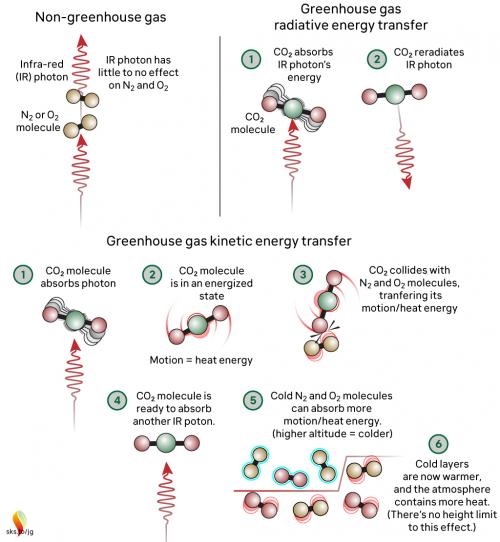
Greenhouse gases in the atmosphere, such as CO2 molecules, absorb some of this IR radiation, then re-emit it in all directions - including back to Earth's surface. The CO2 molecule does not fill up with IR photons, running out of space for any more. Instead, the CO2 molecule absorbs the energy from the IR photon and the photon ceases to be. The CO2 molecule now contains more energy, but that is transient since the molecule emits its own IR photons. Not only that: it's constantly colliding with other molecules such as N2 and O2 in the surrounding air. In those collisions, that excess energy is shared with them. This energy-sharing causes the nearby air to heat up (fig. 2).
Fig. 2: The greenhouse effect in action, showing the interactions between molecules. The interactions happen at all levels of the atmosphere and are constantly ongoing. Graphic: jg.
The capacity for CO2 to absorb photons is almost limitless. The CO2 molecule can also receive energy from collisions with other molecules, and it can lose energy by emitting IR radiation. When a photon is emitted, we’re not bringing a photon out of storage - we are bringing energy out of storage and turning it into a photon, travelling away at the speed of light. So CO2 is constantly absorbing IR radiation, constantly emitting IR radiation and constantly sharing energy with the surrounding air molecules. To understand the role of CO2, we need to consider all these forms of energy storage and transport.
So, where does 'saturation' get used in climate change contrarianism? The most common way they try to frame things is to claim that IR emitted from the surface, in the wavelengths where CO2 absorbs, is all absorbed fairly close to the surface. Therefore, the story continues, adding more CO2 can’t make any more difference. This is inaccurate through omission, because either innocently or deliberately, it ignores the rest of the picture, where energy is constantly being exchanged with other molecules by collisions and CO2 is constantly emitting IR radiation. This means that there is always IR radiation being emitted upwards by CO2 at all levels in the atmosphere. It might not have originated from the surface, but IR radiation is still present in the wavelengths that CO2 absorbs and emits. When emitted in the upper atmosphere, it can and will be lost to space.
When you include all the energy transfers related to the CO2 absorption of IR radiation – the transfer to other molecules, the emission, and both the upward and downward energy fluxes at all altitudes - then we find that adding CO2 to our current atmosphere acts to inhibit the transfer of radiative energy throughout that atmosphere and, ultimately, into space. This will lead to additional warming until the amount of energy being lost to space matches what is being received. This is precisely what is happening.
The myth reproduced at the top – incorrectly stating an analogy with roof insulation in that each unit has less of an effect - is misleading. Doubling CO2 from 280 ppm to 560 ppm will cause a few degrees of warming. Doubling again (560 to 1130 ppm) will cause a similar amount of additional warming, and so on. Many doublings later there may be a point where adding more CO2 has little effect, but recent work has cast serious doubt on that (He et al. 2023). But we are a long, long way from reaching that point and in any case we do not want to go anywhere near it! One doubling will be serious enough.
Finally, directly observing the specific, global radiative forcing caused by well-mixed greenhouse gases has - to date - proven elusive. This is because of irregular, uncalibrated or limited areal measurements. But very recently, results have been published regarding the deep reinterrogation of years of data (2003-2021) from the Atmospheric Infrared Sounder (AIRS) instrument on NASA's Aqua Satellite (Raghuraman et al. 2023). The work may well have finally cracked the long-standing issue of how to make finely detailed, consistent wavelength-specific measurements of outgoing long-wave radiation from Earth into space. As such, it has opened the way to direct monitoring of the radiative impact (i.e. forcing + feedback) of greenhouse gas concentration changes, thereby complimenting the Keeling Curve - the longstanding dataset of measured CO2 concentrations, down at the planet's surface.
Note: Several people in addition to John Mason were involved with updating this basic level rebuttal, namely Bob Loblaw, Ken Rice and John Garrett (jg).
Last updated on 31 December 2023 by John Mason. View Archives































 Arguments
Arguments





































Sgt_Wookie92 @574,
You present a 1,000-word denialist essay on why an increase in atmospheric CO2 will not reduce IR out into space. It is an interesting polemic as it does quite a good job of addressing to some extent all the various descriptions of the GHG mechanisms, descriptions both actual and through analogy. It is however, as described by Eclectic @575, a pack of nonsense.
I could go through paragraph by paragraph if you wish. A blow-by-blow account would be required as the central misconception the denialist employs isn't presented entirely within any single paragraph. Perhaps it would be easier to describe the GHG mechanism and allow to pick out the crazy talk for yourself.
....
The planet surface emits IR in the waveband roughly 5μ to 50μ with the peak at 15μ. The profile is dependent on temperature. If there were no GHGs, all that IR would shoot off into space. But the GHGs actually capture pretty-much all of this surface-emitted IR, the energy converted into waggles in GHGs and almost all of those waggles, through collisions, are converted into thermal energy.
But GHGs also go waggly because of those numerous collisions and that ensures the GHGs will effectively emit just as much IR as it is receives. CO2 absorbs/emits at 2.9μ, 4.3μ and 15μ but only the 15μ operates as the atmosphere/planet is too cold for the shorter wavebands.
And at 15μ, CO2 is the only GHG operating so all the IR at 15μ that reaches space will all be emitted by CO2. The amount of 15μ IR is now depentent on the temperature of the CO2 emitting it out into space. For that the CO2 needs a clear shot at space, high enough so the CO2 above it is no longer a complete blanket. Presently that altitude-with-a-clear-shot-to-space is up in the cold upper troposphere. The graphic below shows that CO2 temperature is far lower than the surface temperature. (The black trace is measured, the red is modelled by MODTRAN, an on-line model from UoC.)
If more CO2 is added to the atmosphere, that altitude-with-a-clear-shot-to-space will get higher (CO2 is well-mixed up to 50km, well up into the stratosphere) and, while that altitude remains below the tropopause, the clear-shot temperature will get colder so less IR will be emitted into space. (The very central part of the 15μ waveband does have a higher clear-shot altitude above the tropopause and is seen in the graphic as a little spike. That spike will grow with additional CO2 while the size of the surrounding dip(s) will continue to increase. See Zhong & Haig 2013).
....
Most of the nonsense set out within the denialist polemic should be understandable given this description (although I'd happily expand on individual points, perhaps some brickbats to lob back at the denialist). I'd just add here that some may not be acquainted with Feldman et al (2015) which is the paper that measured CO2's IR on the "Great Plains and North Slope of Alaska."
Thank you very much for your responses, 575 Eclectic & 576 MA Rodger.
Sgt_Wookie , I take my hat off to MA Rodger for his short description of the physics. Not a full exposition, but nicely succinct. And for myself, I have taken a copy of his graph of infrared energy leaving the planet ~ it's a fine demonstration of the Atmospheric IR windows and the absorption effect of water, carbon dioxide, ozone, and methane.
Your denialist friend is probably so heavily invested in his Motivated Reasoning that he's not persuadable to change his mind (or his attitude), so there's little point in trying to educate him. Though I'm not knowing the circumstances of your "interaction" with him, nevertheless it's the case that most online interactions have a number of silent observers ~ and they benefit when denialist nonsense gets contradicted. But I am sure you're already aware of that . . . and aware of the old saying regarding: ".... when good men do nothing."
IMO the explanation is much too complex.
In the lower atmosphere, absorption is saturated, that's true.
But in the highest layer it is not, because the molecule density is too low. So there is ample room to increase absorbtion there by increasing CO2 density. The highest layer is the pane of the proverbial greenhouse.
Old Arrhenius used in his paper 1896 the concept of the "radiating layer", which is of course the top layer.
dien:
Please explain exactly what you mean when you say "In the lower atmosphere, absorption is saturated". I have yet to hear a decent definition of "saturated" where this is true.
dien @579,
I will be more wide-ranging with my questioning of you than Bob Loblaw @580 although I perhaps understand what you are trying to say.
Can you tell us which "explanation" you are describing as being "much too complex"?
When you use the term "molecule density", to which molecules are you referring, all air molecules or just CO2?
Are you referring to Arrhenius (1896) 'On the influence of carbonic acid in the air upon the temperature of the ground'? and if so, where doe he use the term "radiating layer"? Arrhenius talks of an "emitting layer" but this is not the CO2 emitting layer and thus not what you term "the top layer."
You will note that I don't ask about your meaning of "saturated" although this does not directly apply to CO2's GHG effect being saturated. I feel your overly awkward explanation is essentially correct. That is, if by 'saturation' you meaning that all radiation emitted by CO2 is absorbed and fails to escape to space, this is mainly true in the lower atmosphere. From there, an increase in CO2 (which is well mixed within the atmosphere to a height of perhaps 50km) will increase emission/absorption in the lower atmosphere as well as the upper. (So it is not just "in the highest layer" where there is "ample room to increase absorbtion there by increasing CO2 density.") In so doing, the altitude where all CO2 radiation is absorbed (thus in your words "saturated") does have "ample room to increase" in altitude and, if that increase is still within the troposphere, it will thus to decrease in temperature. It is this decreasing temperature (or to be more exact, decreasing net temperature) which determines whether the GHG effect from increasing CO2 is saturated or not.
@ 580 and 581.
First thx for investing thought about it, which may make things clearer.
I would like to refer to the reference to Angströms experiment in the first part of the "advanced debunking". Not all outgoing IR is absorbed, there is this absorption window (see https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6174548/) . But a certain IR band is absorbed by CO2, and for this band, the absorption lenght is in the order of magnitude of some meters at sea level. This is what I meant with "saturated".
BTW, this is the first point of the myth, which is actually not true, as stated above, because only the IR outside the transmission window will be absorbed.
So the heat energy has to propagate via multiple absorptions to the top layer. You are right, insofar having more CO2 molecules will already hamper this propagation process. My image for this is a stack of frosted glass, each representing one absorption length. The IR will be absorbed and re-emitted in all directions by each pane. To add CO2 means then to add a lot more of those panes. And those will increase the ratio between input and output power.
I have my difficulties with the statement, that the increase of the CO2 concentration pushes the emission layer up, where it is colder, where therefore less heat will be emitted. For me it's the other way around: because more "frosted glass panes" alias absorption lengths are stacked within the atmosphere, we have less power arriving at the top and therefore its temperature will be lower.
This - the lower top atmosphere temperature - is only the case as long as the earth is still warming up. In a new equilibrium, a couple of years after we hopefully managed to keep the CO2-level steady, the top atmosphere will be as warm as before, because it will emit exactly all incoming power. Only of course the surface temperature will be much higher than before.
With the word "complex", I tried to express a certain diffuse discontent with the explanation above. It has actually not explained, how in earth the heat, having been absorbed in the first meter or so, manages to reach the top layer in 50 km or so height nonetheless. Only if we give this explanation, we can make the effect of the additional absorption plausible.
The water metaphor is not bad, but it is a boundary - and conservation-of-energy argument. To use the actual propagation mechanism between the boundaries would imho more enlightening.
Dlen @582 , I am puzzled by what you say in your fourth-to-last paragraph.
Surely, regardless of the number of "frosted glass panes", the power arriving (upwards) at the top must always be the same* and equal to the solar heat entering the system (the system being the sub-TOA system i.e. the planet minus the stratosphere).
Of course, the same* will be very slightly more - or less - according to whether the planet is cooling or warming in transition to a new equilibrium surface temperature.
dlen @582,
The difficulty I have with this discussion is that it is attempting to provide an analogy for the GHG mechanism, something which can never be exact because if it were, it would be too complex when its puropse is to be simple to understand.
You say "So the heat energy has to propagate via multiple absorptions to the top layer" with CO2 acting to "hamper this propagation process."
This is not the best of wording. It is true that the planet sheds energy solely by radiation, something like 240Wm^-2 to be in equilibrium. Yet within the planet's energy flows, very little of this outward energy is 'propagated' from the net radiative energy flux from the surface. The surface is only radiating a net 60Wm^-2, of which 40Wm^-2 is the radiation passing through the "the transmission window" (so plays no part in the GHG mechanism) leaving just 20Wm^-2 which "has to propagate via multiple absorptions to the top layer." Joining this surface radiative energy flux as it 'propagates' upward is 100Wm^-2 of convective and insensible heat transport from the surface as well as 80Wm^-2 from direct solar heating of the atmosphere to yield the full 200Wm^-2 being radiated from the atmosphere out into space. And in being able to radiate at atmospheric temperatures, CO2 does not "hamper" the process but instead assists it.
Your fouth-last paragraph is entirely wrong. It is not the CO2 which warms the atmosphere (ie the troposphere) and determines its temperature profile. The temperature profile (lapse rate) is well balanced so as to hold convection back from running amok. (We would live in an interesting surface environment without this balance!!) The temperature profile (as opposed to the temperature) is certainly not determined by radiation.
The planet surface and atmosphere does of course have to warm because an increase in CO2 results in it emitting into space from higher cooler parts of the atmosphere. While CO2 is well mixed up to perhaps 50km, the effective emission altitude for CO2 is nothing like that high - more like 10km. And while the whole climate system (up to the tropopause) will warm as a result of increased CO2 to allow the radiative balance to be restored, the flux within the CO2 waveband will still remain smaller than previously, while the flux elsewhere (where the effective emission altitude remains unaffected) will be greater.
@ ma rodger, #584: Thx for the lengthy answer and the little lecture about heat pathways. I got the impression, before developing ad hoc heat propagation models, I should have done some reading about convective-radiative heat transfer in the atmosphere.
So, if I interprete your post correctly, the main transport mechanism to the effective emission height is convection, with radiation from the soil being nearly insignificant. This should be named as such in the explanation. It is not very complicated, not hard to understand.
The effective emission height is rising. This is sufficiently well explained in the text. What is not explained is, why it is colder in higher levels of the atmosphere. I see two aspects here:
1. adiabatic expansion of rising air packets. This is a general explanation for the lapse rate. The rising air packet uses up internal energy for expansion work.
2. After the earth has been warmed sufficiently, the now higher effective emission layer will not be colder anymore. The rising air packets will start with a higher temperature = internal energy and so will be warmer even after having risen to a higher effective emission layer.
Dien @585 ,
heat rises through the atmosphere by radiation, convection, and latent heat changes (water phase-change). Consult the famous "Trenberth cartoon" to see the proportions of these.
The lapse rate can be viewed in the simplest term, as like the multiple layers of clothing you wear on a cold day ~ the thermodynamic heat flow across the temperature gradient, from warm innermost layer to coolest outermost layer. You can look at the complexities of each mechanism: but the ultimate effect is a simple gradient, from planetary surface up to the effective radiation "escape" layer.
To whom may be interested,
I've recently come across this physics study after running into it from this article.
The study's demonstrating the saturation argument with respect to thermal radiative flux from the earth to outer space when compared to varying CO2 and water vapor concentration, effectively countering the Greenhouse Effect argument being proposed in this page.
aoeu @587,
The paper you link-to is apparently co-authored by the physicist and climate change denier William Happer so if it did conclude with the message presented on the article you link-to on the rogue planetoid Wattsupia, there would be no real surprise. Happer has been the author of quite a bit of arrant ninsense on te subject of climatology.
The Wattsupian take on the paper runs:-
However, this account of the paper is total nonsense (so par for the course for Wattsupia). The paper actually concludes by saying it finds 2xCO2 without feedbacks would increase global temperature by +1.4ºC and with feedback (constant relative humidity) under clear-sky conditions by +2.2ºC, this finding close to other studies.
MA Rodger is correct, of course. Nick Stokes commented in WTFIUWTW: "They basically redid Manabe’s calc from 1967 and got a very similar answer. It has been known for that time that if you do a 1-D calc with just radiative, including simple hypotheses about the response of water vapor, you get CS in that range. But there is a lot more going on."
Aoeu @587 , permit me to add my 2 cents as well.
The WUWT article is a "nothingburger" - and worse.
The WUWT editor has given a completely fallacious headline. (Typical for WUWT) The article is based on a paper - unpublished - by two scientists, one of whom is the eminent Dr Happer. It is claimed that Happer's paper has been knocked back by three major journals . . . and reading the paper soon shows why a scientific journal would not bother to publish this paper.
You will see from the above comments by MA Rodger and Tom Dayton, that the Happer paper comes out with a CO2-doubling Climate Sensitivity of 2.2 degreesC . . . a finding which is wildly misrepresented by the WUWT editor. This 2.2C sensitivity is slightly below the 3.0 figure which is a fairer "average" of sensitivity assessments (based on paleo and modern empirical evidence). So really nothing new there.
The paper has two weaknesses. It makes no allowance for cloud effects (the paper is a "clear sky" model). And as a minor point, it uses constant relative humidity in its modeling. Apart from that, I have no particular criticism to make . . . other than the humorous one where a typographical error shows "temperature region" where "temperate region" was meant ;-)
Clearly the Happer paper is not worth publishing.
Sadly, the blog WUWT is trumpeting this paper to the skies (excuse pun). WattsUpWithThat and its denialist clientele are always desperate to make much of anything at all which comes even within a million miles of casting some doubt on mainstream climate science.
Aoeu, have a look at the WUWT comments column below the article. There are all sorts of frothing-at-the-mouth comments . . . that this new paper overthrows all previous climate science / disproves the Greenhouse effect / exposes the incompetence & corrupt criminality of all the thousands of climate scientists worldwide. Etcetera. All the usual WattsUpWithThat nonsense and crackpot lunacy. But among all the madness, you will find a few pearls of wisdom by the genuine scientist Nick Stokes (who is thoroughly hated by the usual WUWT clientele).
We can expect the Happer paper will be a Nine Day Wonder in many parts of the bloggy Deniosphere . . . until they abandon it for the Next New Thing (by Lord Christopher Monckton or whoever). It is all very entertaining . . . but it ain't science.
Aoeu - a small addition :-
Dr Happer's paper seems more than a tad confused about the meaning of "saturation" in regard to Greenhouse gasses & climate.
I am not sure that the WUWT editor actually managed to read the paper. Certainly many (most?) of the subsequent WUWT commenters didn't.
So now we have this: https://arxiv.org/pdf/2006.03098.pdf
Change anyone's mind? It probably should cause deep reflection at a minimum.
[DB] In order to facilitate a proper dialog, it is incumbent upon you to first provide your synthesisized interpretation of that paper for others to then weigh in on. What-about-this-ism does not advance the conversation.
Further, operating multiple accounts here is forbidden by this site's Comments Policy.
Oops. I was on the wrong page … Happer's work is already logged in.
ChezProvence:
As you can see if you read the comments directly above yours, this paper was rejected from peer reviewed ournals and is poorly written. I doubt anyones mind will be changed.
The co2 saturation is obvious, it's the difference between wearing five feet of blankets and five miles. Insulation is proportional to 1/thickness^2 so obviously anything beyond the first few feet of the hundreds of meters of co2 in the air is irrelevant.
This is obvious to a small child so the global warming advocates are just not beleving what they are saying.
Diminishing returns on insulation
Ingrahammark7 @ 595 / 596 :
You have missed the mark - by a mile.
What a pity you did not read the Original Post. And in particular, have a think about the second diagram there.
Ingraham . . . how can you educate yourself, if you don't read?
( Hint: the atmosphere is a gas, not a wool blanket. )
Ingrahammark7 @595/596,
While it is correct that conductive insulation is dependent on the temperature gradient through the insulation and the less the gradient, the less the heat loss, the OP above makes clear the mechnism of CO2 insulation does not work in that manner. So only a badly taught "small child" would hold the views you suggest.
We are not taking blankets where an additional blanket will diminish the outward energy flow by stretching the distance between inside and outside temperatures and thus reducing the temperature gradient within the insulative blankets. The level of radiative energy flying about the atmosphere is not diminished by adding extra CO2. Also, the temperature gradient up through the atmosphere is not determined by CO2. The workings of the greenhouse effect is explained by the OP in its Basic/Intermediate/Advanced versions.
Mind, your logic about R-values and blankets is also in error. The logic of the R-value is used to assess the insulation of buildings. It is useful because buildings have thermostats. The planet Earth has no thermostat (other than CO2 levels). And there is no thermostat in a bed. Add too many blankets and the occupant will die of hyperthermia.
Eclectic, the second diagram is incoherent, if you think adding to a five mile blanket adds any insulation then then there is something wrong. This does not take any math, or diagrams, if insulation worked in a linear way then it would lead to absurd outcomes and everyone would already be dead.
"Add too many blankets and the occupant will die of hyperthermia" this is what I want you to see demonstrate in real life. You are not going to arbitrarily raise heat by adding blankets. Insulation only raises temperature a few degrees in the first few inches and after that is negligible.
Here is my request, since the graph I provide would just show the same as I already gave. Chart the incremental temperature insulation from different thicknesses of co2. Whether you call It insulation or something else is irrelevant. You chart should be utterly absurd because you have to explain why the first few co2 cause the entire effect and once you get to hundreds or thousands of feet which exist a doubling does nothing.
"Also, the temperature gradient up through the atmosphere is not determined by CO2." In case you were expecting a response to this- that statement is unrelated to the discussion but it nonethless further refutes your point. The temperature gradient in the atmosphere is determined by pressure. The greenhouse effect would break this relationship and raise temperatures at lower altitudes where diminishing returns is less.